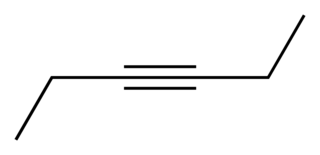In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.
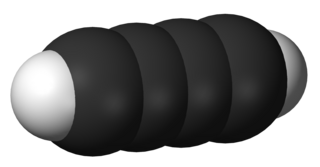
Diacetylene (also known as butadiyne) is the organic compound with the formula C4H2. It is the simplest compound containing two triple bonds. It is first in the series of polyynes, which are of theoretical but not of practical interest.

Propyne (methylacetylene) is an alkyne with the chemical formula CH3C≡CH. It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.
Sulfur oxide refers to many types of sulfur and oxygen containing compounds such as SO, SO2, SO3, S7O2, S6O2, S2O2, etc.

1-Butyne is an organic compound with the formula CH3CH2C≡CH. It is a terminal alkyne. The compound is a common terminal alkyne substrate in diverse studies of catalysis. It is a colorless combustible gas.

2-Butyne (dimethylacetylene, crotonylene or but-2-yne) is an alkyne with chemical formula CH3C≡CCH3. Produced artificially, it is a colorless, volatile, pungent liquid at standard temperature and pressure.
Alcohol most commonly refers to:
Lead sulfide refers to two compounds containing lead and sulfur:
Metal-organic compounds are a class of chemical compounds that contain metals and organic ligands, but lacking direct metal-carbon bonds. Metal β-diketonates, metal alkoxides, metal dialkylamides, transition metal carboxylate complexes, metal acetylacetonates, and metal phosphine complexes are representative members of this class. Some of metal-organic compounds confer solubility in organic solvents or volatility. Compounds with these properties find applications in materials science for metal organic vapor deposition (MOCVD) or sol-gel processing. Precise definitions of metal-organic compound may vary, however the term may describe:
The molecular formula C4H6 (molar mass: 54.09 g/mol) may refer to:
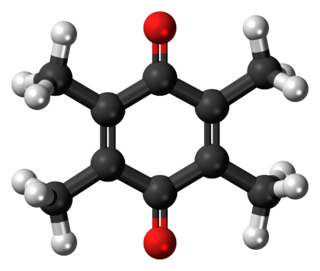
Duroquinone is an organic oxidant (C6(CH3)4O2). It is related to 1,4-benzoquinone by replacement of four H centres with methyl (Me) groups. The C10O2 core of this molecule is planar with two pairs of C=O and C=C bonds.
Dichlorophenols (DCPs) are any of several chemical compounds which are derivatives of phenol containing two chlorine atoms. There are six isomers:
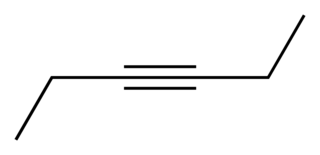
3-Hexyne is the organic compound with the formula C2H5CCC2H5. This colorless liquid is one of three isomeric hexynes. 3-Hexyne forms with 5-decyne, 4-octyne, and 2-butyne a series of symmetric alkynes. It is a reagent in organometallic chemistry.
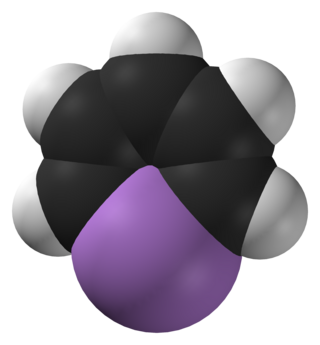
Arsabenzene (IUPAC name: arsinine) is an organoarsenic heterocyclic compound with the chemical formula C5H5As. It belongs to a group of compounds called heteroarenes that have the general formula C5H5E (E= N, P, As, Sb, Bi).
Tribromide is the anion with the chemical formula Br3−, or salts containing it:
Urushibara nickel is a nickel-based hydrogenation catalyst. It is a heterogeneous catalyst, comparable to Raney nickel. Urushibara nickel is however not pyrophoric. For most hydrogenations, it performs comparably to W-7 grade Raney nickel.
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.

Hexafluorocyclobutene is the organofluorine compound with the formula (CF2)2(CF)2. A colorless gas, it is a precursor to a variety of compounds, including squaric acid. Hexafluorocyclobutene is prepared in two steps from chlorotrifluoroethylene. The thermal dimerization gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane. Dechlorination of the latter gives hexafluorocyclobutene:
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.