| |
| Names | |
|---|---|
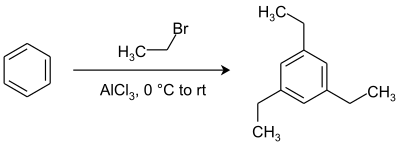
| Preferred IUPAC name 1,3,5-Triethylbenzene | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ECHA InfoCard | 100.002.744 |
| EC Number |
|
| MeSH | 1,3,5-triethylbenzene |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C12H18 | |
| Molar mass | 162.27 g·mol −1 |
| Appearance | colorless liquid [1] |
| Density | 0.862 g·cm−3 [1] |
| Melting point | −66.5 °C (−87.7 °F; 206.7 K) [2] |
| Boiling point | 215 °C (419 °F; 488 K) [1] |
| practically insoluble [1] | |
| Solubility in ethanol, diethyl ether | soluble [3] |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H413 | |
| P264, P273, P280, P302+P352, P305+P351+P338, P332+P313 | |
| Flash point | 76 °C (169 °F; 349 K) [1] |
| Safety data sheet (SDS) | [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
1,3,5-Triethylbenzene is a chemical compound of the group of aromatic hydrocarbons.