Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term tetrahedranes is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.

Cyclobutadiene is an organic compound with the formula C4H4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.

2-Butanol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. Its structural isomers are 1-butanol. isobutanol, and tert-butanol. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-2-butanol and (S)-(+)-2-butanol. It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture.

Biphenyl is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one hydrogen may use the prefixes xenyl or diphenylyl.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
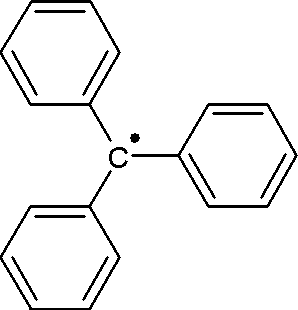
The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trityl radical has been heavily exploited.
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.
Pivalic acid is a carboxylic acid with a molecular formula of (CH3)3CCO2H. This colourless, odiferous organic compound is solid at room temperature. A common abbreviation for the pivalyl or pivaloyl group (t-BuC(O)) is Piv and for pivalic acid (t-BuC(O)OH) is PivOH.
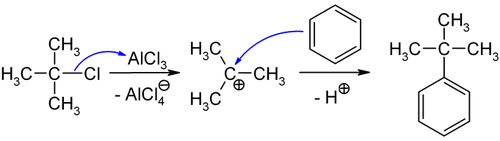
tert-Butyl chloride is the organochloride with the formula (CH3)3CCl. It is a colorless, flammable liquid. It is sparingly soluble in water, with a tendency to undergo hydrolysis to the corresponding tert-butyl alcohol. It is produced industrially as a precursor to other organic compounds.

Durene, or 1,2,4,5-tetramethylbenzene, is an organic compound with the formula C6H2(CH3)4. It is a colourless solid with a sweet odor. The compound is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and isodurene (1,2,3,5-tetramethylbenzene). Durene has an unusually high melting point (79.2 °C), reflecting its high molecular symmetry.
tert-Butyl isocyanide is an organic compound with the formula Me3CNC (Me = methyl, CH3). It is an isocyanide, commonly called isonitrile or carbylamine, as defined by the functional group C≡N-R. tert-Butyl isocyanide, like most alkyl isocyanides, is a reactive colorless liquid with an extremely unpleasant odor. It forms stable complexes with transition metals and can insert into metal-carbon bonds.

Neophyl chloride, C6H5C(CH3)2CH2Cl, is a halogenated organic compound with unusual nucleophilic substitution properties. Neophyl chloride is used to form a versatile organolithium reagent, neophyl lithium, by reaction with lithium.

Tetra-tert-butylethylene is a hypothetical organic compound, a hydrocarbon with formula C18H36, or ((H3C−)3C−)2C=C(−C(−CH3)3)2. As the name indicates, its molecular structure can be viewed as an ethylene molecule H2C=CH2 with the four hydrogens replaced by tert-butyl −C(−CH3)3 groups.

tert-Butylthiol, also known as 2-methylpropane-2-thiol, 2-methyl-2-propanethiol, tert-butyl mercaptan (TBM), and t-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol is used as an odorant for natural gas, which is otherwise odorless. It may also have been used as a flavoring agent.

tert-Butyl peroxybenzoate (TBPB) a chemical compound from the group of peresters (compounds containing the general structure R1-C(O)OO-R2) which contains a phenyl group as R1 and a tert-butyl group as R2. It is often used as a radical initiator in polymerization reactions, such as the production of LDPE from ethylene, and for crosslinking, such as for unsaturated polyester resins.

Glybuzole is a hypoglycaemic medicine, mainly used to treat diabetes mellitus type 2. It is an oral antidiabetic drug (OAD), when administered in the right dose it will help bring the blood glycose level down by stimulating the insulin production. Similar medicines are glimepiride, glipizide, glibenclamide, gliclazide, and gliquidone.

The C4-benzenes are a class of organic aromatic compounds which contain a benzene ring and four other carbon atoms. There are three tetramethylbenzenes, six dimethylethylbenzenes, three diethylbenzenes, three isopropylmethylbenzenes, three n-propylmethylbenzenes and four butylbenzenes. The saturated compounds have formula C10H14 and molecular weight 134.22 g/mol. C4-benzenes are found in petroleum. Petrol (gasoline) can contain 5-8% C4-benzenes.

n-Butylbenzene is the organic compound with the formula C6H5C4H9. Of two isomers of butylbenzene, n-butylbenzene consists of a phenyl group attached to the 1 position of a butyl group. It is a slightly greasy, colorless liquid.
Fulvio Cacace was an Italian chemist.

sec-Butylbenzene is an organic compound classified as an aromatic hydrocarbon. Its structure consists of a benzene ring substituted with a sec-butyl group. It is a flammable colorless liquid which is nearly insoluble in water but miscible with organic solvents.