A request that this article title be changed to 2-Chloro-2-methylpropane is under discussion . Please do not move this article until the discussion is closed. |
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2-Chloro-2-methylpropane | |||
| Other names 1,1-dimethylethyl chloride 1-chloro-1,1-dimethylethane chlorotrimethylmethane trimethylchloromethane t-butyl chloride | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.334 | ||
| EC Number |
| ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1127 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C4H9Cl | |||
| Molar mass | 92.57 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.851 g/ml | ||
| Melting point | −26 °C (−15 °F; 247 K) | ||
| Boiling point | 51 °C (124 °F; 324 K) | ||
| Sparingly soluble in water, miscible with alcohol and ether | |||
| Vapor pressure | 34.9 kPa (20 °C) | ||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Danger | |||
| H225 | |||
| P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −9 °C (16 °F; 264 K) (open cup) −23 °C (closed cup) | ||
| 540 °C (1,004 °F; 813 K) | |||
| Related compounds | |||
Related alkyl halides | tert-Butyl bromide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
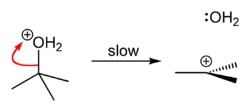
tert-Butyl chloride is the organochloride with the formula (CH3)3CCl. It is a colorless, flammable liquid. It is sparingly soluble in water, with a tendency to undergo hydrolysis to the corresponding tert-butyl alcohol. It is produced industrially as a precursor to other organic compounds. [1]