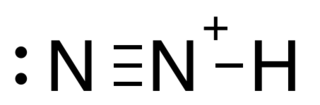Hydrogen cyanide is a chemical compound with the formula HCN and structural formula H−C≡N. It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature.
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the cation [H3O]+, also written as H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, H+) to the surrounding water molecules (H2O). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base. Three main structures for the aqueous proton have garnered experimental support: the Eigen cation, which is a tetrahydrate, H3O+(H2O)3, the Zundel cation, which is a symmetric dihydrate, H+(H2O)2, and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4. Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. For this reason, it has been suggested that wherever possible, the symbol H+(aq) should be used instead of the hydronium ion.

Astrochemistry is the study of the abundance and reactions of molecules in the universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar System and the interstellar medium. The study of the abundance of elements and isotope ratios in Solar System objects, such as meteorites, is also called cosmochemistry, while the study of interstellar atoms and molecules and their interaction with radiation is sometimes called molecular astrophysics. The formation, atomic and chemical composition, evolution and fate of molecular gas clouds is of special interest, because it is from these clouds that solar systems form.

A protostar is a very young star that is still gathering mass from its parent molecular cloud. This is the earliest phase in the process of stellar evolution. For a low-mass star, it lasts about 500,000 years. The phase begins when a molecular cloud fragment first collapses under the force of self-gravity and an opaque, pressure supported core forms inside the collapsing fragment. It ends when the infalling gas is depleted, leaving a pre-main-sequence star, which contracts to later become a main-sequence star at the onset of hydrogen fusion producing helium.

The trihydrogen cation or protonated molecular hydrogen is a cation with formula H+
3, consisting of three hydrogen nuclei (protons) sharing two electrons.
Vibrational circular dichroism (VCD) is a spectroscopic technique which detects differences in attenuation of left and right circularly polarized light passing through a sample. It is the extension of circular dichroism spectroscopy into the infrared and near infrared ranges.
GRW +70 8247 is a white dwarf star located 42 light-years from Earth in the constellation Draco. With a magnitude of about 13 it is visible only through a large telescope.
Hydrogen isocyanide is a chemical with the molecular formula HNC. It is a minor tautomer of hydrogen cyanide (HCN). Its importance in the field of astrochemistry is linked to its ubiquity in the interstellar medium.

Moganite is an oxide mineral with the chemical formula SiO2 (silicon dioxide) that was discovered in 1976. It was initially described as a new form of silica from specimens found in the Barranco de Medio Almud, in the municipality of Mogán on the island of Gran Canaria, in the Canary Islands (Spain), receiving in a later work the name derived from this locality. In 1994 the International Mineralogical Association decided to disapprove it as a valid mineral, since it was considered indistinguishable from quartz. Subsequent studies allowed the IMA to rectify it in 1999, accepting it as a mineral species. It has the same chemical composition as quartz, but a different crystal structure.
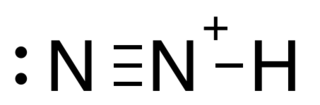
Diazenylium is the chemical N2H+, an inorganic cation that was one of the first ions to be observed in interstellar clouds. Since then, it has been observed for in several different types of interstellar environments, observations that have several different scientific uses. It gives astronomers information about the fractional ionization of gas clouds, the chemistry that happens within those clouds, and it is often used as a tracer for molecules that are not as easily detected (such as N2). Its 1–0 rotational transition occurs at 93.174 GHz, a region of the spectrum where Earth's atmosphere is transparent and it has a significant optical depth in both cold and warm clouds so it is relatively easy to observe with ground-based observatories. The results of N2H+ observations can be used not only for determining the chemistry of interstellar clouds, but also for mapping the density and velocity profiles of these clouds.

The cyano radical (or cyanido radical) is a radical with molecular formula CN, sometimes written •CN. The cyano radical was one of the first detected molecules in the interstellar medium, in 1938. Its detection and analysis was influential in astrochemistry. The discovery was confirmed with a coudé spectrograph, which was made famous and credible due to this detection. ·CN has been observed in both diffuse clouds and dense clouds. Usually, CN is detected in regions with hydrogen cyanide, hydrogen isocyanide, and HCNH+, since it is involved in the creation and destruction of these species (see also Cyanogen).
In astronomy, the velocity dispersion (σ) is the statistical dispersion of velocities about the mean velocity for a group of astronomical objects, such as an open cluster, globular cluster, galaxy, galaxy cluster, or supercluster. By measuring the radial velocities of the group's members through astronomical spectroscopy, the velocity dispersion of that group can be estimated and used to derive the group's mass from the virial theorem. Radial velocity is found by measuring the Doppler width of spectral lines of a collection of objects; the more radial velocities one measures, the more accurately one knows their dispersion. A central velocity dispersion refers to the σ of the interior regions of an extended object, such as a galaxy or cluster.

Chromium(I) hydride, systematically named chromium hydride, is an inorganic compound with the chemical formula (CrH)
n. It occurs naturally in some kinds of stars where it has been detected by its spectrum. However, molecular chromium(I) hydride with the formula CrH has been isolated in solid gas matrices. The molecular hydride is very reactive. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.

Iron(I) hydride, systematically named iron hydride and poly(hydridoiron) is a solid inorganic compound with the chemical formula (FeH)
n (also written ([FeH])
n or FeH). It is both thermodynamically and kinetically unstable toward decomposition at ambient temperature, and as such, little is known about its bulk properties.

Calcium monohydride is a molecule composed of calcium and hydrogen with formula CaH. It can be found in stars as a gas formed when calcium atoms are present with hydrogen atoms.

Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride.

Vela Molecular Ridge is a molecular cloud complex in the constellations Vela and Puppis. Radio 12CO observations of the region showed the ridge to be composed of several clouds, each with masses 100,000–1,000,000 M☉. This cloud complex lies on the sky in the direction of the Gum Nebula (foreground) and the Carina–Sagittarius Spiral Arm (background). The most important clouds in the region are identified by the letters A, B, C and D, and in fact belong to two different complexes: the clouds A, C and D are located at an average distance of about 700-1000 parsecs and are related to the OB association Vela R2, while cloud B is located at a greater distance, up to 2000 parsecs away, and is physically connected to the extended Vela OB1 association.

Argonium (also called the argon hydride cation, the hydridoargon(1+) ion, or protonated argon; chemical formula ArH+) is a cation combining a proton and an argon atom. It can be made in an electric discharge, and was the first noble gas molecular ion to be found in interstellar space.

Hydroxyaluminium(I), also known as Aluminium(I) hydroxide, is an inorganic chemical with molecular formula AlOH. It consists of aluminium in the +1 oxidation state paired with a single hydroxide. It has been detected as a molecular substance in the envelope of an oxygen-rich red supergiant star, a place where substances containing metals or hydroxides are thought to be rare.