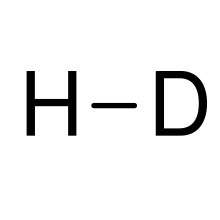 | |
 | |
| Names | |
|---|---|
| IUPAC name Hydrogen deuteride | |
| Systematic IUPAC name | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.034.325 |
| EC Number |
|
PubChem CID | |
| UN number | 1049 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| HD | |
| Molar mass | 3.02204 g mol−1 |
| Melting point | −259 °C (−434.2 °F; 14.1 K) |
| Boiling point | −253 °C (−423.4 °F; 20.1 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H220, H280 | |
| P210, P377, P381, P403, P410+P403 | |
| NFPA 704 (fire diamond) | |
| 571 °C (1,060 °F; 844 K) | |
| Related compounds | |
Related hydrogens | Deuterium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Hydrogen deuteride is an isotopologue of dihydrogen composed of two isotopes of hydrogen: the majority isotope 1H (protium) and 2H (deuterium). Its proper molecular formula is 1H2H, but for simplification, it is usually written as HD.


