External links
| Fields | |
|---|---|
| Concepts | |
| Treatments | |
| Incidents | |
| Related topics | |
Acceptable daily intake or ADI is a measure of the amount of a specific substance (originally applied for a food additive, later also for a residue of a veterinary drug or pesticide) in food or drinking water that can be ingested (orally) daily over a lifetime without an appreciable health risk. [1] ADIs are expressed usually in milligrams (of the substance) per kilograms of body weight per day. [2] [3]
This concept was first introduced in 1961 by the Council of Europe and later, the Joint FAO/WHO Expert Committee on Food Additives (JECFA), a committee maintained by two United Nations bodies: the Food and Agriculture Organization (FAO) and the World Health Organization (WHO). [2]
An ADI value is based on current research, with long-term studies on animals and observations of humans. First, a no-observed-adverse-effect level (NOAEL), [3] [4] the amount of a substance that shows no toxic effects, is determined. Usually the studies are performed with several doses including high doses. In the case of several studies on different effects, the lowest NOAEL is usually taken. Then, the NOAEL (or another point of departure such as a benchmark dose level (BMDL)) is divided by a safety factor, conventionally 100, to account for the differences between test animals and humans (factor of 10) and possible differences in sensitivity between humans (another factor of 10). [3] Safety factors with values other than 100 may be used if information on uncertainty about the value of the point of departure (NOAEL or BMDL) justify it. For instance, if the ADI is based on data from humans the safety factor is usually 10 instead of 100. The ADI is usually given in mg per kg body weight. [5]
The ADI is considered a safe intake level for a healthy adult of normal weight who consumes an average daily amount of the substance in question. Increased safety factors for infants have been discussed, but are not needed, because elimination of chemicals is in fact often more rapid in children and as children generally have higher illness rates than adults, adverse effects caused by food additives can easily be disguised as any number of things children usually experience. It would be far more difficult to argue the case with a healthy adult. [6] The ADI does not take into account allergic reactions that are individual responses rather than dose-dependent phenomena.
The higher the ADI, the larger amounts of a compound are safe for regular ingestion. The concept of tolerable daily intake is often used for unwanted contaminants or other chemicals.
The ADI concept can be understood as a measure to indicate the toxicity from long-term exposure to repeated ingestion of chemical compounds in foods (present and/or added), as opposed to acute toxicity.
The threshold limit value (TLV) of a chemical substance is a level to which it is believed a worker can be exposed day after day for a working lifetime without adverse effects.
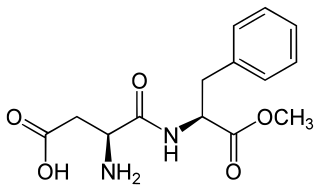
Aspartame is an artificial non-saccharide sweetener 200 times sweeter than sucrose and is commonly used as a sugar substitute in foods and beverages. It is a methyl ester of the aspartic acid/phenylalanine dipeptide with brand names NutraSweet, Equal, and Canderel. Aspartame was approved by the US Food and Drug Administration (FDA) in 1974, and then again in 1981, after approval was revoked in 1980.

Toxicology is a scientific discipline, overlapping with biology, chemistry, pharmacology, and medicine, that involves the study of the adverse effects of chemical substances on living organisms and the practice of diagnosing and treating exposures to toxins and toxicants. The relationship between dose and its effects on the exposed organism is of high significance in toxicology. Factors that influence chemical toxicity include the dosage, duration of exposure, route of exposure, species, age, sex, and environment. Toxicologists are experts on poisons and poisoning. There is a movement for evidence-based toxicology as part of the larger movement towards evidence-based practices. Toxicology is currently contributing to the field of cancer research, since some toxins can be used as drugs for killing tumor cells. One prime example of this is ribosome-inactivating proteins, tested in the treatment of leukemia.

Sucralose is an artificial sweetener and sugar substitute. As the majority of ingested sucralose is not metabolized by the body, it adds no calories. In the European Union, it is also known under the E number E955. It is produced by chlorination of sucrose, selectively replacing three of the hydroxy groups—in the C1 and C6 positions of the fructose portion and the C4 position of the glucose portion—to give a 1,6-dichloro-1,6-dideoxyfructose–4-chloro-4-deoxygalactose disaccharide. Sucralose is about 600 times sweeter than sucrose, three times as sweet as both aspartame and acesulfame potassium, and twice as sweet as sodium saccharin.

Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell (cytotoxicity) or an organ such as the liver (hepatotoxicity). Sometimes the word is more or less synonymous with poisoning in everyday usage.
In toxicology, the lethal dose (LD) is an indication of the lethal toxicity of a given substance or type of radiation. Because resistance varies from one individual to another, the "lethal dose" represents a dose at which a given percentage of subjects will die. The lethal concentration is a lethal dose measurement used for gases or particulates. The LD may be based on the standard person concept, a theoretical individual that has perfectly "normal" characteristics, and thus not apply to all sub-populations.
A reference dose is the United States Environmental Protection Agency's maximum acceptable oral dose of a toxic substance, "below which no adverse noncancer health effects should result from a lifetime of exposure". Reference doses have been most commonly determined for pesticides. The EPA defines an oral reference dose as:
[A]n estimate, with uncertainty spanning perhaps an order of magnitude, of a daily oral exposure to the human population that is likely to be without an appreciable risk of deleterious effects during a lifetime.
The threshold limit value (TLV) is believed to be a level to which a worker can be exposed per shift in the worktime without adverse effects. Strictly speaking, TLV is a reserved term of the American Conference of Governmental Industrial Hygienists (ACGIH). TLVs issued by the ACGIH are the most widely accepted occupational exposure limits both in the United States and most other countries. However, it is sometimes loosely used to refer to other similar concepts used in occupational health and toxicology, such as acceptable daily intake (ADI) and tolerable daily intake (TDI). Concepts such as TLV, ADI, and TDI can be compared to the no-observed-adverse-effect level (NOAEL) in animal testing, but whereas a NOAEL can be established experimentally during a short period, TLV, ADI, and TDI apply to human beings over a lifetime and thus are harder to test empirically and are usually set at lower levels. TLVs, along with biological exposure indices (BEIs), are published annually by the ACGIH.

Mirex is an organochloride that was commercialized as an insecticide and later banned because of its impact on the environment. This white crystalline odorless solid is a derivative of cyclopentadiene. It was popularized to control fire ants but by virtue of its chemical robustness and lipophilicity it was recognized as a bioaccumulative pollutant. The spread of the red imported fire ant was encouraged by the use of mirex, which also kills native ants that are highly competitive with the fire ants. The United States Environmental Protection Agency prohibited its use in 1976. It is prohibited by the Stockholm Convention on Persistent Organic Pollutants.
Exposure assessment is a branch of environmental science and occupational hygiene that focuses on the processes that take place at the interface between the environment containing the contaminant of interest and the organism being considered. These are the final steps in the path to release an environmental contaminant, through transport to its effect in a biological system. It tries to measure how much of a contaminant can be absorbed by an exposed target organism, in what form, at what rate and how much of the absorbed amount is actually available to produce a biological effect. Although the same general concepts apply to other organisms, the overwhelming majority of applications of exposure assessment are concerned with human health, making it an important tool in public health.
The no-observed-adverse-effect level (NOAEL) denotes the level of exposure of an organism, found by experiment or observation, at which there is no biologically or statistically significant increase in the frequency or severity of any adverse effects of the tested protocol. In drug development, the NOAEL of a new drug is assessed in laboratory animals, such as mice, prior to initiation of human trials in order to establish a safe clinical starting dose in humans. The OECD publishes guidelines for Preclinical Safety Assessments, in order to help scientists discover the NOAEL.
Patulin is an organic compound classified as a polyketide. It is a white powder soluble in acidic water and in organic solvents. It is a lactone that is heat-stable, so it is not destroyed by pasteurization or thermal denaturation. However, stability following fermentation is lessened. It is a mycotoxin produced by a variety of molds, in particular, Aspergillus and Penicillium and Byssochlamys. Most commonly found in rotting apples, the amount of patulin in apple products is generally viewed as a measure of the quality of the apples used in production. In addition, patulin has been found in other foods such as grains, fruits, and vegetables. Its presence is highly regulated.

Disodium 5'-ribonucleotides or I+G, E number E635, is a flavor enhancer which is synergistic with glutamates in creating the taste of umami. It is a mixture of disodium inosinate (IMP) and disodium guanylate (GMP) and is often used where a food already contains natural glutamates or added monosodium glutamate (MSG). It is primarily used in flavored noodles, snack foods, chips, crackers, sauces and fast foods. It is produced by combining the sodium salts of the natural compounds guanylic acid (E626) and inosinic acid (E630).
The maximum residue limit is the maximum amount of pesticide residue that is expected to remain on food products when a pesticide is used according to label directions, that will not be a concern to human health.
Toxic equivalency factor (TEF) expresses the toxicity of dioxins, furans and PCBs in terms of the most toxic form of dioxin, 2,3,7,8-TCDD. The toxicity of the individual congeners may vary by orders of magnitude.

Carbophenothion also known as Stauffer R 1303 as for the manufacturer, Stauffer Chemical, is an organophosphorus chemical compound. It was used as a pesticide for citrus fruits under the name of Trithion. Carbophenothion was used as an insecticide and acaricide. Although not used anymore it is still a restricted use pesticide in the United States. The chemical is identified in the US as an extremely hazardous substance according to the Emergency Planning and Community Right-to-Know Act.
Tolerable daily intake (TDI) refers to the daily amount of a chemical that has been assessed safe for human being on long-term basis. Originally acceptable daily intake (ADI) was introduced in 1961 to define the daily intake of a food additive which, during the entire lifetime, appears to be without appreciable risk. For contaminants and other foreign chemicals not used intentionally, the term TDI is often preferred. Both ADI and TDI are usually assessed based on animal experiments, and it is most often hundreds of times lower than the dose causing no observable adverse effect (NOAEL) in the most sensitive tested animal species. Because the confounding factors may vary depending on the quality of data and the type of adverse effect, TDI values are not good estimates of the harmfulness of chemicals, and must be considered administrative tools to set allowable limits for chemicals, rather than scientific measures. The threshold limit value (TLV) of a chemical substance is a level to which it is believed a worker can be exposed day after day for a working lifetime without adverse effects.
Dietary exposure assessments in the United States involve the evaluation of dietary consumption and chemical residue data while taking into consideration additional factors that may affect a specified population of interest or sensitive population. The process of conducting a dietary exposure assessment involves the determination of the chemical residues on a particular food or foods and the calculation of the dietary exposure to these chemicals based on consumption data for the specified food or foods. A dietary exposure assessment allows a comparison to a relevant health standard such as the acceptable daily intake(ADI), the acute reference dose.
Tolerable weekly intake (TWI) estimates the amount per unit body weight of a potentially harmful substance or contaminant in food or water that can be ingested over a lifetime without risk of adverse health effects. TWI is generally preceded by "provisional" to indicate insufficient data exists, increasing uncertainty. The term TWI should be reserved for when there is a well-established and internationally accepted tolerance, backed by sound and uncontested data. Although similar in concept to tolerable daily intake (TDI), which is of the same derivation of acceptable daily intakes (ADIs), TWI accounts for contaminants that do not clear the body quickly and may accumulate within the body over a period of time. An example is heavy metals such as arsenic, cadmium, lead, and mercury. The concept of TWI takes into account daily variations in human consumption patterns.
Occupational toxicology is the application of toxicology to chemical hazards in the workplace. It focuses on substances and conditions that people may be exposed to in workplaces, including inhalation and dermal exposures, which are most prevalent when discussing occupational toxicology. These environmental and individual exposures can impact health, and there is a focus on identifying early adverse affects that are more subtle than those presented in clinical medicine.
Threshold dose is the minimum dose of drug that triggers minimal detectable biological effect in an animal. At extremely low doses, biological responses are absent for some of the drugs. The increase in dose above threshold dose induces an increase in the percentage of biological responses. Several benchmarks have been established to describe the effects of a particular dose of drug in a particular species, such as NOEL(no-observed-effect-level), NOAEL(no-observed-adverse-effect-level) and LOAEL(lowest-observed-adverse-effect-level). They are established by reviewing the available studies and animal studies. The application of threshold dose in risk assessment safeguards the participants in human clinical trials and evaluates the risks of chronic exposure to certain substances. However, the nature of animal studies also limits the applicability of experimental results in the human population and its significance in evaluating potential risk of certain substances. In toxicology, there are some other safety factors including LD50, LC50 and EC50.