
Cooking, also known as cookery or professionally as the culinary arts, is the art, science and craft of using heat to make food more palatable, digestible, nutritious, or safe. Cooking techniques and ingredients vary widely, from grilling food over an open fire, to using electric stoves, to baking in various types of ovens, reflecting local conditions. Cooking is an aspect of all human societies and a cultural universal.

A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.

Nicotinamide (INN, BAN UK) or niacinamide (USAN US) is a form of vitamin B3 found in food and used as a dietary supplement and medication. As a supplement, it is used orally (swallowed by mouth) to prevent and treat pellagra (niacin deficiency). While nicotinic acid (niacin) may be used for this purpose, nicotinamide has the benefit of not causing skin flushing. As a cream, it is used to treat acne, and has been observed in clinical studies to improve the appearance of aging skin by reducing hyperpigmentation and redness. It is a water-soluble vitamin.
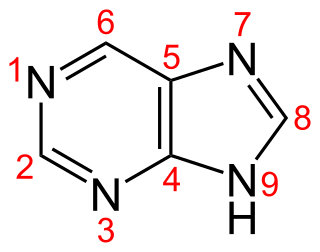
Purine is a heterocyclic aromatic organic compound that consists of two rings fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature.
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom (=N−). It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.

Adenine is a purine nucleotide base. It is one of the four nucleobases in the nucleic acids of DNA, the other three being guanine (G), cytosine (C), and thymine (T). Adenine derivatives have various roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate (ATP) and the cofactors nicotinamide adenine dinucleotide (NAD), flavin adenine dinucleotide (FAD) and Coenzyme A. It also has functions in protein synthesis and as a chemical component of DNA and RNA. The shape of adenine is complementary to either thymine in DNA or uracil in RNA.

Nucleotide bases are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nucleic acids. The ability of nucleobases to form base pairs and to stack one upon another leads directly to long-chain helical structures such as ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Five nucleobases—adenine (A), cytosine (C), guanine (G), thymine (T), and uracil (U)—are called primary or canonical. They function as the fundamental units of the genetic code, with the bases A, G, C, and T being found in DNA while A, G, C, and U are found in RNA. Thymine and uracil are distinguished by merely the presence or absence of a methyl group on the fifth carbon (C5) of these heterocyclic six-membered rings. In addition, some viruses have aminoadenine (Z) instead of adenine. It differs in having an extra amine group, creating a more stable bond to thymine.
The nitrite ion has the chemical formula NO−
2. Nitrite is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid.
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a "deliquescent crystal or wax-like solid with a pungent, sweet, corn-like, nutty odour".
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system. Many simple aromatic rings have trivial names. They are usually found as substructures of more complex molecules. Typical simple aromatic compounds are benzene, indole, and pyridine.
Basic aromatic rings are aromatic rings in which the lone pair of electrons of a ring-nitrogen atom is not part of the aromatic system and extends in the plane of the ring. This lone pair is responsible for the basicity of these nitrogenous bases, similar to the nitrogen atom in amines. In these compounds the nitrogen atom is not connected to a hydrogen atom. Basic aromatic compounds get protonated and form aromatic cations under acidic conditions. Typical examples of basic aromatic rings are pyridine or quinoline. Several rings contain basic as well as non-basic nitrogen atoms, e.g. imidazole and purine.
Thiazole, or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).

The ANRORC mechanism in organic chemistry describes a special type of substitution reaction. ANRORC stands for Addition of the Nucleophile, Ring Opening, and Ring Closure in nucleophilic attack on ring systems and it helps to explain product formation and distribution in some nucleophilic substitutions especially in heterocyclic compounds. It is widely used in medicinal chemistry.

In molecular genetics, a DNA adduct is a segment of DNA bound to a cancer-causing chemical. This process could lead to the development of cancerous cells, or carcinogenesis. DNA adducts in scientific experiments are used as biomarkers of exposure. They are especially useful in quantifying an organism's exposure to a carcinogen. The presence of such an adduct indicates prior exposure to a potential carcinogen, but it does not necessarily indicate the presence of cancer in the subject animal.

Hexazine is a hypothetical allotrope of nitrogen composed of 6 nitrogen atoms arranged in a ring-like structure analogous to that of benzene. As a neutrally charged species, it would be the final member of the azabenzene (azine) series, in which all of the methine groups of the benzene molecule have been replaced with nitrogen atoms. The two last members of this series, hexazine and pentazine, have not been observed, although all other members of the azine series have.

Nucleic acid analogues are compounds which are analogous to naturally occurring RNA and DNA, used in medicine and in molecular biology research. Nucleic acids are chains of nucleotides, which are composed of three parts: a phosphate backbone, a pentose sugar, either ribose or deoxyribose, and one of four nucleobases. An analogue may have any of these altered. Typically the analogue nucleobases confer, among other things, different base pairing and base stacking properties. Examples include universal bases, which can pair with all four canonical bases, and phosphate-sugar backbone analogues such as PNA, which affect the properties of the chain . Nucleic acid analogues are also called xeno nucleic acids and represent one of the main pillars of xenobiology, the design of new-to-nature forms of life based on alternative biochemistries.

Harmane (harman) is a heterocyclic amine found in a variety of foods including coffee, sauces, and cooked meat. It is also present in tobacco smoke.

Heterocyclic amines (HCAs) are a group of chemical compounds, many of which can be formed during cooking. They are found in meats that are cooked to the "well done" stage, in pan drippings and in meat surfaces that show a brown or black crust. Epidemiological studies show associations between intakes of heterocyclic amines and cancers of the colon, rectum, breast, prostate, pancreas, lung, stomach, and esophagus, and animal feeding experiments support a causal relationship. The U.S. Department of Health and Human Services Public Health Service labeled several heterocyclic amines as likely carcinogens in its 13th Report on Carcinogens. Changes in cooking techniques reduce the level of heterocyclic amines.

PhIP (2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) is one of the most abundant heterocyclic amines (HCAs) in cooked meat. PhIP is formed at high temperatures from the reaction between creatine or creatinine, amino acids, and sugar. PhIP formation increases with the temperature and duration of cooking and also depends on the method of cooking and the variety of meat being cooked. The U.S. Department of Health and Human Services National Toxicology Program has declared PhIP as "reasonably anticipated to be a human carcinogen". International Agency for Research on Cancer (IARC), part of World Health Organization, has classified PhIP as IARC Group 2B carcinogen. There is sufficient evidence in experimental animals, as well as in vitro models, for the carcinogenicity of PhIP.
















