
Benzoic acid is a white solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring with a carboxyl substituent. The benzoyl group is often abbreviated "Bz", thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –C6H5CO. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source.
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or by undesirable chemical changes. In general, preservation is implemented in two modes, chemical and physical. Chemical preservation entails adding chemical compounds to the product. Physical preservation entails processes such as refrigeration or drying. Preservative food additives reduce the risk of foodborne infections, decrease microbial spoilage, and preserve fresh attributes and nutritional quality. Some physical techniques for food preservation include dehydration, UV-C radiation, freeze-drying, and refrigeration. Chemical preservation and physical preservation techniques are sometimes combined.

A soft drink is any water-based flavored drink, usually but not necessarily carbonated, and typically including added sweetener. Flavors used can be natural or artificial. The sweetener may be a sugar, high-fructose corn syrup, fruit juice, a sugar substitute, or some combination of these. Soft drinks may also contain caffeine, colorings, preservatives and other ingredients.

Vitamin C is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical "serum" ingredient to treat melasma and wrinkles on the face. It is used to prevent and treat scurvy. Vitamin C is an essential nutrient involved in the repair of tissue, the formation of collagen, and the enzymatic production of certain neurotransmitters. It is required for the functioning of several enzymes and is important for immune system function. It also functions as an antioxidant. Most animals are able to synthesize their own vitamin C. However, apes and monkeys, most bats, some rodents, and certain other animals must acquire it from dietary sources.

Ethyl carbamate (also called urethane) is an organic compound with the formula CH3CH2OC(O)NH2. It is an ester of carbamic acid and a white solid. Despite its name, it is not a component of polyurethanes. Because it is a carcinogen, it is rarely used, but naturally forms in low quantities in many types of fermented foods and drinks.

Potassium benzoate (E212), the potassium salt of benzoic acid, is a food preservative that inhibits the growth of mold, yeast and some bacteria. It works best in low-pH products, below 4.5, where it exists as benzoic acid.

Sodium benzoate also known as benzoate of soda is the sodium salt of benzoic acid, widely used as a food preservative (with an E number of E211) and a pickling agent. It appears as a white crystalline chemical with the formula C6H5COONa.

Shasta Beverages is an American soft drink manufacturer that markets a value-priced soft drink line with a wide variety of soda flavors, as well as a few drink mixers, under the brand name Shasta. The company name is derived from Mount Shasta in northern California and the associated Shasta Springs.

Texas Pete is a brand of hot sauce in the United States developed and manufactured by the TW Garner Food Company in Winston-Salem, North Carolina. TW Garner was founded by Thad W. Garner in 1929. As of 2022, Texas Pete is the seventh-best selling hot sauce in the U.S., according to Instacart, an online grocery service.

Cranberry juice is the liquid juice of the cranberry – a fruit recognized for its bright red color, tart taste, and versatility for product manufacturing. Major cranberry products include cranberry juice, dried cranberry, cranberry sauce, frozen cranberry, cranberry powder, and dietary supplements containing cranberry extracts.

Allura Red AC is a red azo dye that goes by several names, including FD&C Red 40. It is used as a food dye and has the E number E129.

The nutrition facts label is a label required on most packaged food in many countries, showing what nutrients and other ingredients are in the food. Labels are usually based on official nutritional rating systems. Most countries also release overall nutrition guides for general educational purposes. In some cases, the guides are based on different dietary targets for various nutrients than the labels on specific foods.
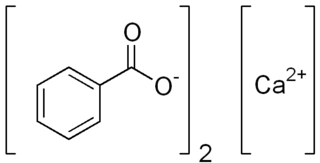
Calcium benzoate refers to the calcium salt of benzoic acid. When used in the food industry as a preservative, its E number is E213 ; it is approved for use as a food additive in the EU, USA and Australia and New Zealand.
Fruit2O, formerly manufactured by Kraft, is a lightly flavored, non-carbonated water beverage introduced in 1999. Fruit2o was introduced to compete not only with the bottled water market but also with the soft drink market. Sunny Delight Beverages purchased the Veryfine Products line from Kraft in 2007.
Cocaine, also known as No Name, is a highly caffeinated energy drink distributed by Redux Beverages. It contains more caffeine than rival energy drinks Red Bull and Rockstar, symbolized by three and a half steer heads on the label. Aside from caffeine, the label claims 750 milligrams of taurine, another common ingredient found in many energy drinks.
Patulin is an organic compound classified as a polyketide. It is a white powder soluble in acidic water and in organic solvents. It is a lactone that is heat-stable, so it is not destroyed by pasteurization or thermal denaturation. However, stability following fermentation is lessened. It is a mycotoxin produced by a variety of molds, in particular, Aspergillus and Penicillium and Byssochlamys. Most commonly found in rotting apples, the amount of patulin in apple products is generally viewed as a measure of the quality of the apples used in production. In addition, patulin has been found in other foods such as grains, fruits, and vegetables. It's presence is highly regulated.

A dough conditioner, flour treatment agent, improving agent or bread improver is any ingredient or chemical added to bread dough to strengthen its texture or otherwise improve it in some way. Dough conditioners may include enzymes, yeast nutrients, mineral salts, oxidants and reductants, bleaching agents and emulsifiers. They are food additives combined with flour to improve baking functionality. Flour treatment agents are used to increase the speed of dough rising and to improve the strength and workability of the dough.
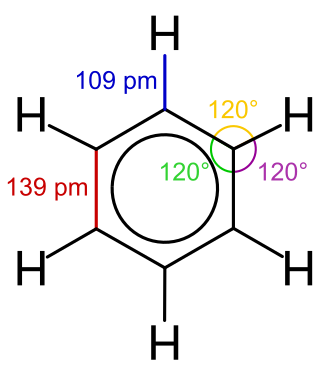
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.

4-Methylimidazole is a heterocyclic organic chemical compound with molecular formula H
3C–C
3H
3N
2 or C
4H
6N
2. It is formally derived from imidazole through replacement of the hydrogen in position 4 by a methyl group. It is a slightly yellowish solid.















