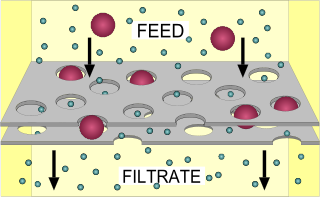
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms.

In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".
An electrolyte is a medium containing ions that is electrically conducting through the movement of ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly through the solvent. Solid-state electrolytes also exist. In medicine, the term electrolyte refers to the substance that is dissolved.

In chemistry, solubility is ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.

Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. Adsorption is a surface phenomenon, while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term sorption encompasses both processes, while desorption is the reverse of it.
Palladium hydride is metallic palladium that contains a substantial quantity of hydrogen within its crystal lattice. Despite its name, it is not an ionic hydride but rather an alloy of palladium with metallic hydrogen that can be written PdHx. At room temperature, palladium hydrides may contain two crystalline phases, α and β. Pure α-phase exists at x < 0.017 whereas pure β-phase is realised for x > 0.58; intermediate x values correspond to α-β mixtures.

In chemistry, absorption is a physical or chemical phenomenon or a process in which atoms, molecules or ions enter some bulk phase – liquid or solid material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume, not by the surface.
Desorption is the physical process where a previously adsorbed substance is released from a surface. This happens when a molecule gains enough energy to overcome the activation barrier or the bounding energy that keeps it in the surface.

Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances.
Adsorption refrigeration was invented by Michael Faraday in 1821, even though the basis of artificial modern refrigeration dates back to 1748 with William Cullen experiments. More on history of refrigeration can be found in the paragraph Refrigeration Research on page Refrigeration. Adsorption, sometimes is referred to solid sorption.
Inner sphere complex is a type of surface complex that refers to the surface chemistry changing a water-surface interface to one without water molecules bridging a ligand to the metal ion. Formation of inner sphere complexes occurs when ions bind directly to the surface with no intervening water molecules. These types of surface complexes are restricted to ions that have a high affinity for surface sites and include specifically adsorbed ions that can bind to the surface through covalent bonding.

Sodium polyacrylate, also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula [−CH2−CH(CO2Na)−]n and has broad applications in consumer products. This super-absorbent polymer (SAP) has the ability to absorb 100 to 1000 times its mass in water. Sodium polyacrylate is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain. Sodium polyacrylate is a chemical polymer made up of chains of acrylate compounds. It contains sodium, which gives it the ability to absorb large amounts of water. When dissolved in water, it forms a thick and transparent solution due to the ionic interactions of the molecules. Sodium polyacrylate has many favorable mechanical properties. Some of these advantages include good mechanical stability, high heat resistance, and strong hydration. It has been used as an additive for food products including bread, juice, and ice cream.

An absorption refrigerator is a refrigerator that uses a heat source to provide the energy needed to drive the cooling process. The system uses two coolants, the first of which performs evaporative cooling and is then absorbed into the second coolant; heat is needed to reset the two coolants to their initial states. The principle can also be used to air-condition buildings using the waste heat from a gas turbine or water heater. Using waste heat from a gas turbine makes the turbine very efficient because it first produces electricity, then hot water, and finally, air-conditioning—trigeneration. Absorption refrigerators are commonly used in recreational vehicles (RVs), campers, and caravans because the heat required to power them can be provided by a propane fuel burner, by a low-voltage DC electric heater or by a mains-powered electric heater. Unlike more common vapor-compression refrigeration systems, an absorption refrigerator can be produced with no moving parts other than the coolants.
Moisture analysis covers a variety of methods for measuring moisture content in solids, liquids, or gases. For example, moisture is a common specification in commercial food production. There are many applications where trace moisture measurements are necessary for manufacturing and process quality assurance. Trace moisture in solids must be known in processes involving plastics, pharmaceuticals and heat treatment. Fields that require moisture measurement in gasses or liquids include hydrocarbon processing, pure semiconductor gases, bulk pure or mixed gases, dielectric gases such as those in transformers and power plants, and natural gas pipeline transport.
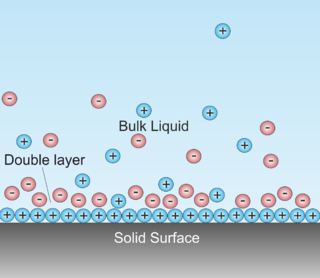
A double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". (-> this description of DL is not right, at least concerning the electrode/electrolyte interface. Here DL refers to charge separation at the interface with the electrode possessing negative charge and the electrolyte positive charge. The two layers are separated by some molecular distance. The two layers mentioned in above description are all at the electrolyte side.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
Sorption enhanced water gas shift (SEWGS) is a technology that combines a pre-combustion carbon capture process with the water gas shift reaction (WGS) in order to produce a hydrogen rich stream from the syngas fed to the SEWGS reactor.










