In chemistry, a disulfide refers to a functional group with the structure R−S−S−R′. The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. The connection is a persulfide, in analogy to its congener, peroxide (R−O−O−R′), but this terminology is rarely used, except in reference to hydrodisulfides.

Hydrogen sulfide is the chemical compound with the formula H
2S. It is a colorless chalcogen hydride gas with the characteristic foul odor of rotten eggs. It is very poisonous, corrosive, and flammable.

The green sulfur bacteria (Chlorobiaceae) are a family of obligately anaerobic photoautotrophic bacteria. Together with the non-photosynthetic Ignavibacteriaceae, they form the phylum Chlorobi.

Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate (SO42–) as terminal electron acceptor, reducing it to hydrogen sulfide (H2S). Therefore, these sulfidogenic microorganisms "breathe" sulfate rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration.

Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: anions and organic polysulfides. Anions have the general formula S2−
n. These anions are the conjugate bases of the hydrogen polysulfides H2Sn. Organic polysulfides generally have the formulae RSnR, where R = alkyl or aryl.
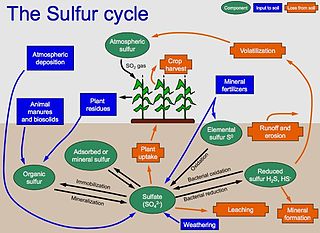
The sulfur cycle is the collection of processes by which sulfur moves between rocks, waterways and living systems. Such biogeochemical cycles are important in geology because they affect many minerals. Biochemical cycles are also important for life because sulfur is an essential element, being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes.

Sodium sulfide is the chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both are colorless water-soluble salts that give strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, which smells like rotten eggs. Some commercial samples are specified as Na2S·xH2O, where a weight percentage of Na2S is specified. Commonly available grades have around 60% Na2S by weight, which means that x is around 3. Such technical grades of sodium sulfide have a yellow appearance owing to the presence of polysulfides. These grades of sodium sulfide are marketed as 'sodium sulfide flakes'. Although the solid is yellow, solutions of it are colorless.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
In enzymology, a cytochrome-c3 hydrogenase (EC 1.12.2.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a sulfur oxygenase/reductase (EC 1.13.11.55) is an enzyme that catalyzes the chemical reaction

Sulfite reductases (EC 1.8.99.1) are enzymes that participate in sulfur metabolism. They catalyze the reduction of sulfite to hydrogen sulfide and water. Electrons for the reaction are provided by a dissociable molecule of either NADPH, bound flavins, or ferredoxins.

Thiosulfate dehydrogenase is an enzyme that catalyzes the chemical reaction:
Sulfur is metabolized by all organisms, from bacteria and archaea to plants and animals. Sulfur is reduced or oxidized by organisms in a variety of forms. The element is present in proteins, sulfate esters of polysaccharides, steroids, phenols, and sulfur-containing coenzymes.

Hydrogen disulfide is the inorganic compound with the formula H2S2. This hydrogen chalcogenide is a pale yellow volatile liquid with a camphor-like odor. It decomposes readily to hydrogen sulfide (H2S) and elemental sulfur.

Flavocytochrome c sulfide dehydrogenase, also known as Sulfide-cytochrome-c reductase (flavocytochrome c) (EC 1.8.2.3), is an enzyme with systematic name hydrogen-sulfide:flavocytochrome c oxidoreductase. It is found in sulfur-oxidising bacteria such as the purple phototrophic bacteria Allochromatium vinosum. This enzyme catalyses the following chemical reaction:

Trisulfane is the inorganic compound with the formula H2S3. It is a pale yellow volatile liquid with a camphor-like odor. It decomposes readily to hydrogen sulfide (H2S) and elemental sulfur. It is produced by distillation of the polysulfane oil obtained by acidification of polysulfide salts.

Sodium polysulfide is a general term for salts with the formula Na2Sx, where x = 2 to 5. The species Sx2−, called polysulfide anions, include disulfide (S22−), trisulfide (S32−), tetrasulfide (S42−), and pentasulfide (S52−). In principle, but not in practice, the chain lengths could be longer. The salts are dark red solids that dissolve in water to give highly alkaline and corrosive solutions. In air, these salts oxidize, and they evolve hydrogen sulfide by hydrolysis.
Sodium tetrasulfide is an inorganic compound with the formula Na2S4. It is a yellow-orange solid that dissolved with hydrolysis in water. They are precursors to some specialty polymers and intermediates in prototypes of the sodium-sulfur battery.
Sulfide:quinone reductase is an enzyme with systematic name sulfide:quinone oxidoreductase. This enzyme catalyses the following chemical reaction