This article's lead section may need to be rewritten. The reason given is: Per MOS:INTRO, "avoid difficult-to-understand terminology" in the lead.(September 2022) |

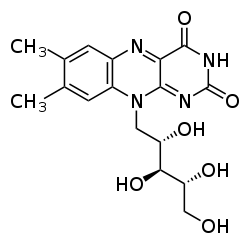
Flavins (from Latin flavus, "yellow") refers generally to the class of organic compounds containing the tricyclic heterocycle isoalloxazine or its isomer alloxazine, and derivatives thereof. The biochemical source of flavin is the yellow B vitamin riboflavin. The flavin moiety is often attached with an adenosine diphosphate to form flavin adenine dinucleotide (FAD), and, in other circumstances, is found as flavin mononucleotide (or FMN), a phosphorylated form of riboflavin. It is in one or the other of these forms that flavin is present as a prosthetic group in flavoproteins. Despite the similar names, flavins (with "i") are chemically and biologically distinct from the flavanoids (with "a"), and the flavonols (with "o").
Contents
The flavin group is capable of undergoing oxidation-reduction reactions, and can accept either one electron in a two-step process or two electrons at once. Reduction is made with the addition of hydrogen atoms to specific nitrogen atoms on the isoalloxazine ring system:

In aqueous solution, flavins are yellow-coloured when oxidized, taking a red colour in the semi-reduced anionic state or blue in the neutral (semiquinone) state, and colourless when totally reduced. [1] The oxidized and reduced forms are in fast equilibrium with the semiquinone (radical) form, shifted against the formation of the radical: [2]
- Flox + FlredH2 ⇌ FlH•
where Flox is the oxidized flavin, FlredH2 the reduced flavin (upon addition of two hydrogen atoms) and FlH• the semiquinone form (addition of one hydrogen atom).
In the form of FADH2, it is one of the cofactors that can transfer electrons to the electron transfer chain.

