Related Research Articles
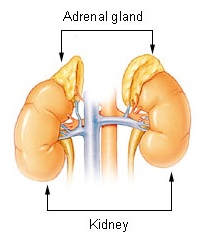
The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.

The endocrine system is a messenger system in an organism comprising feedback loops of hormones that are released by internal glands directly into the circulatory system and that target and regulate distant organs. In vertebrates, the hypothalamus is the neural control center for all endocrine systems.
Adrenocorticotropic hormone is a polypeptide tropic hormone produced by and secreted by the anterior pituitary gland. It is also used as a medication and diagnostic agent. ACTH is an important component of the hypothalamic-pituitary-adrenal axis and is often produced in response to biological stress. Its principal effects are increased production and release of cortisol and androgens by the cortex and medulla of the adrenal gland, respectively. ACTH is also related to the circadian rhythm in many organisms.

Cushing's syndrome is a collection of signs and symptoms due to prolonged exposure to glucocorticoids such as cortisol. Signs and symptoms may include high blood pressure, abdominal obesity but with thin arms and legs, reddish stretch marks, a round red face due to facial plethora, a fat lump between the shoulders, weak muscles, weak bones, acne, and fragile skin that heals poorly. Women may have more hair and irregular menstruation. Occasionally there may be changes in mood, headaches, and a chronic feeling of tiredness.

The hypothalamic–pituitary–adrenal axis is a complex set of direct influences and feedback interactions among three components: the hypothalamus, the pituitary gland, and the adrenal glands. These organs and their interactions constitute the HPA axis.

The adrenal cortex is the outer region and also the largest part of the adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.
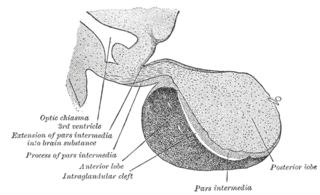
A major organ of the endocrine system, the anterior pituitary is the glandular, anterior lobe that together with the posterior lobe makes up the pituitary gland (hypophysis) which, in humans, is located at the base of the brain, protruding off the bottom of the hypothalamus.

Cortisol is a steroid hormone, in the glucocorticoid class of hormones and a stress hormone. When used as a medication, it is known as hydrocortisone.

Addison's disease, also known as primary adrenal insufficiency, is a rare long-term endocrine disorder characterized by inadequate production of the steroid hormones cortisol and aldosterone by the two outer layers of the cells of the adrenal glands, causing adrenal insufficiency. Symptoms generally come on slowly and insidiously and may include abdominal pain and gastrointestinal abnormalities, weakness, and weight loss. Darkening of the skin in certain areas may also occur. Under certain circumstances, an adrenal crisis may occur with low blood pressure, vomiting, lower back pain, and loss of consciousness. Mood changes may also occur. Rapid onset of symptoms indicates acute adrenal failure, which is a clinical emergency. An adrenal crisis can be triggered by stress, such as from an injury, surgery, or infection.
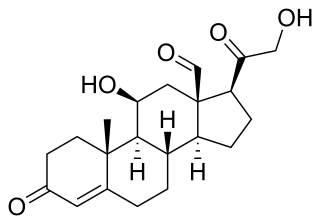
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.

Adrenal insufficiency is a condition in which the adrenal glands do not produce adequate amounts of steroid hormones. The adrenal glands—also referred to as the adrenal cortex—normally secrete glucocorticoids, mineralocorticoids, and androgens. These hormones are important in regulating blood pressure, electrolytes, and metabolism as a whole. Deficiency of these hormones leads to symptoms ranging from abdominal pain, vomiting, muscle weakness and fatigue, low blood pressure, depression, mood and personality changes to organ failure and shock. Adrenal crisis may occur if a person having adrenal insufficiency experiences stresses, such as an accident, injury, surgery, or severe infection; this is a life-threatening medical condition resulting from severe deficiency of cortisol in the body. Death may quickly follow.
Corticotropes are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.

Endocrine glands are ductless glands of the endocrine system that secrete their products, hormones, directly into the blood. The major glands of the endocrine system include the pineal gland, pituitary gland, pancreas, ovaries, testicles, thyroid gland, parathyroid gland, hypothalamus and adrenal glands. The hypothalamus and pituitary glands are neuroendocrine organs.
3β-Hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD) is an enzyme that catalyzes the biosynthesis of the steroid progesterone from pregnenolone, 17α-hydroxyprogesterone from 17α-hydroxypregnenolone, and androstenedione from dehydroepiandrosterone (DHEA) in the adrenal gland. It is the only enzyme in the adrenal pathway of corticosteroid synthesis that is not a member of the cytochrome P450 family. It is also present in other steroid-producing tissues, including the ovary, testis and placenta. In humans, there are two 3β-HSD isozymes encoded by the HSD3B1 and HSD3B2 genes.
The ACTH test is a medical test usually requested and interpreted by endocrinologists to assess the functioning of the adrenal glands' stress response by measuring the adrenal response to adrenocorticotropic hormone or another corticotropic agent such as tetracosactide or alsactide (Synchrodyn). ACTH is a hormone produced in the anterior pituitary gland that stimulates the adrenal glands to release cortisol, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and aldosterone.
Hypoadrenocorticism in dogs, or, as it is known in people, Addison's disease, is an endocrine system disorder that occurs when the adrenal glands fail to produce enough hormones for normal function. The adrenal glands secrete glucocorticoids such as cortisol and mineralocorticoids such as aldosterone; when proper amounts of these are not produced, the metabolic and electrolyte balance is upset. Mineralocorticoids control the amount of potassium, sodium, and water in the body. Hypoadrenocorticism is fatal if left untreated.

Adrenal gland disorders are conditions that interfere with the normal functioning of the adrenal glands. Your body produces too much or too little of one or more hormones when you have an adrenal gland dysfunction. The type of issue you have and the degree to which it affects your body's hormone levels determine the symptoms.
Glucocorticoid remediable aldosteronism also describable as aldosterone synthase hyperactivity, is an autosomal dominant disorder in which the increase in aldosterone secretion produced by ACTH is no longer transient.

Amphenone B, or simply amphenone, also known as 3,3-bis(p-aminophenyl)butan-2-one, is an inhibitor of steroid hormone and thyroid hormone biosynthesis which was never marketed but has been used as a tool in scientific research to study corticosteroids and the adrenal glands. It acts as competitive inhibitor of 11β-hydroxylase, 17α-hydroxylase, 17,20-lyase, 21-hydroxylase, and 3β-hydroxysteroid dehydrogenase, as well as of cholesterol side-chain cleavage enzyme, thereby inhibiting the production of steroid hormones including glucocorticoids, mineralocorticoids, androgens, and estrogens. In addition, amphenone B inhibits the production of thyroxine by a thiouracil-like mechanism, specifically via inhibition of organic binding of iodine and uptake of iodide by the thyroid gland.

Adrenalism describes the condition of an excessive or substandard secretion of hormones related to the adrenal glands, which are found directly superior to the kidneys. Adrenalism can be further distinguished as hyperadrenalism, referring to the excessive secretion of hormones, and hypoadrenalism, referring to the insufficient secretion of hormones.
References
- 1 2 Genest, J., Biron, P., Koiw, E., Nowaczynski, W., Chretien, M., & Boucher, R. (1961). Adrenocortical hormones in human hypertension and their relation to angiotensin. Circulation Research, 9, 775-791.
- 1 2 3 4 5 6 Shier, D., Butler, J., Lewis, R. “Adrenal Glands.” Hole’s Human Anatomy & Physiology. 12th ed. New York: McGraw-Hill, 2010. 504-508.
- 1 2 3 [Connell, J. M. C., & Davies, E. (2005). The new biology of aldosterone. Journal of Endocrinology, 186, 1-20.]
- ↑ Nelson, D. L., Lehninger, A. L., and Cox, M. M. “Hormones are Chemically Diverse.” Lehninger Principles of Biochemistry. 6th ed. New York: W.H. Freeman, 2013. 908.
- ↑ Sushko, T. A., Gilep, A. A., & Usanov, S. A. (2012) Mechanism of intermolecular interactions of microsomal cytochrome P450s CYP17 and CYP21 involved in steroid hormone biosynthesis. Biochemistry, 77(6), 585-592.
- ↑ Duarte, A., Poderoso, C., Cooke, M., Soria, G., Cornejo Maciel, F., et al. (2012). Mitochondrial fusion is essential for steroid biosynthesis. PLoS ONE, 7(9): e45829.
- ↑ Porterfield, J. R., Thompson, G. B., Young, W. F., et al. (2008). Surgery for cushing’s syndrome: An historical review and recent ten-year experience. World J. Surg., 32, 659-677.
- 1 2 Low, G., & Sahi, K. (2012). Clinical and imaging overview of functional adrenal neoplasms. International Journal of Urology, 19, 697-708.
- 1 2 Winqvist, O., Rorsman, F., & Kampe, O. (2000). Autoimmune adrenal insufficiency. BioDrugs, 13(2), 107-114.
- ↑ Cole, J.T., Mitala, C. M., Kundu, S., Verma, A., Elkind, J.A., Nissim, I., & Cohen, A.S. (2010). Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proceedings of the National Academy of Sciences, 107, 366-371.
- ↑ Butcher, S. K., Killampalli, V., Lascelles, D., Wang, K., Alpar, E. K., & Lord, J. M. (2005). Raised cortisol:DHEAS ratios in the elderly after injury: potential impact upon neutrophil function and immunity. Aging Cell, 4, 319-324.