Calcium channel blockers (CCB), calcium channel antagonists or calcium antagonists are a group of medications that disrupt the movement of calcium through calcium channels. Calcium channel blockers are used as antihypertensive drugs, i.e., as medications to decrease blood pressure in patients with hypertension. CCBs are particularly effective against large vessel stiffness, one of the common causes of elevated systolic blood pressure in elderly patients. Calcium channel blockers are also frequently used to alter heart rate, to prevent peripheral and cerebral vasospasm, and to reduce chest pain caused by angina pectoris.

Piracetam is a drug that has efficacy in cognitive disorders, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia; sources differ on its usefulness for dementia. Piracetam is sold as a medication in many European countries. Sale of piracetam is not illegal in the United States, although it is not regulated nor approved by the FDA, so it is legally sold for research use only.

Dizocilpine (INN), also known as MK-801, is a pore blocker of the NMDA receptor, a glutamate receptor, discovered by a team at Merck in 1982. Glutamate is the brain's primary excitatory neurotransmitter. The channel is normally blocked with a magnesium ion and requires depolarization of the neuron to remove the magnesium and allow the glutamate to open the channel, causing an influx of calcium, which then leads to subsequent depolarization. Dizocilpine binds inside the ion channel of the receptor at several of PCP's binding sites thus preventing the flow of ions, including calcium (Ca2+), through the channel. Dizocilpine blocks NMDA receptors in a use- and voltage-dependent manner, since the channel must open for the drug to bind inside it. The drug acts as a potent anti-convulsant and probably has dissociative anesthetic properties, but it is not used clinically for this purpose because of the discovery of brain lesions, called Olney's lesions (see below), in laboratory rats. Dizocilpine is also associated with a number of negative side effects, including cognitive disruption and psychotic-spectrum reactions. It inhibits the induction of long term potentiation and has been found to impair the acquisition of difficult, but not easy, learning tasks in rats and primates. Because of these effects of dizocilpine, the NMDA receptor pore blocker ketamine is used instead as a dissociative anesthetic in human medical procedures. While ketamine may also trigger temporary psychosis in certain individuals, its short half-life and lower potency make it a much safer clinical option. However, dizocilpine is the most frequently used uncompetitive NMDA receptor antagonist in animal models to mimic psychosis for experimental purposes.

Nimodipine, sold under the brand name Nimotop among others, is a calcium channel blocker used in preventing vasospasm secondary to subarachnoid hemorrhage. It was originally developed within the calcium channel blocker class as it was used for the treatment of high blood pressure, but is not used for this indication.

The subfornical organ (SFO) is one of the circumventricular organs of the brain. Its name comes from its location on the ventral surface of the fornix near the interventricular foramina, which interconnect the lateral ventricles and the third ventricle. Like all circumventricular organs, the subfornical organ is well-vascularized, and like all circumventricular organs except the subcommissural organ, some SFO capillaries have fenestrations, which increase capillary permeability. The SFO is considered a sensory circumventricular organ because it is responsive to a wide variety of hormones and neurotransmitters, as opposed to secretory circumventricular organs, which are specialized in the release of certain substances.

Cinnarizine is an antihistamine and calcium channel blocker of the diphenylmethylpiperazine group. It is prescribed for nausea and vomiting due to motion sickness or other sources such as chemotherapy, vertigo, or Ménière's disease. Cinnarizine is one of the leading causes of drug-induced parkinsonism.

Isradipine is a calcium channel blocker of the dihydropyridine class. It is usually prescribed for the treatment of high blood pressure in order to reduce the risk of stroke and heart attack.

Flunarizine, sold under the brand name Sibelium among others, is a drug classified as a calcium antagonist which is used for various indications. It is not available by prescription in the United States or Japan. The drug was discovered at Janssen Pharmaceutica (R14950) in 1968.
The 5-HT3 receptor belongs to the Cys-loop superfamily of ligand-gated ion channels (LGICs) and therefore differs structurally and functionally from all other 5-HT receptors (5-hydroxytryptamine, or serotonin receptors) which are G protein-coupled receptors. This ion channel is cation-selective and mediates neuronal depolarization and excitation within the central and peripheral nervous systems.
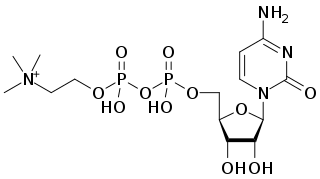
Citicoline (INN), also known as cytidine diphosphate-choline (CDP-choline) or cytidine 5'-diphosphocholine is an intermediate in the generation of phosphatidylcholine from choline, a common biochemical process in cell membranes. Citicoline is naturally occurring in the cells of human and animal tissue, in particular the organs.

Antalarmin (CP-156,181) is a drug that acts as a CRH1 antagonist.

Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects.

Efonidipine (INN) is a dihydropyridine calcium channel blocker marketed by Shionogi & Co. of Japan. It was launched in 1995, under the brand name Landel (ランデル). The drug blocks both T-type and L-type calcium channels. Drug Controller General of India (DCGI) has approved the use of efonidipine in India. It is launched under the brand name "Efnocar".

Dotarizine is a drug used in the treatment of migraine, which acts as a calcium channel blocker, and also as an antagonist at the 5HT2A receptor, and to a lesser extent at the 5HT1A and 5HT2C receptors. The anti-migraine action is thought to be due to its action as a vasodilator, but it also has some anxiolytic effects and blocks amnesia produced by electroconvulsive shock in animals.

5,7-Dihydroxytryptamine (5,7-DHT) is a monoaminergic neurotoxin used in scientific research to decrease concentrations of serotonin in the brain. The mechanism behind this effect is not well understood, but it is speculated to selectively destroy serotonergic neurons, in a manner similar to the dopaminergic neurotoxicity of 6-hydroxydopamine (6-OHDA). What is known is that this compound is in fact not selective in depleting serotonin content, but also depletes norepinephrine. To selectively deplete serotonin stores, it is commonly administered in conjunction with desmethylimipramine (desipramine), which inhibits the norepinephrine transporter.

Indeloxazine (INN) is an antidepressant and cerebral activator that was marketed in Japan and South Korea by Yamanouchi Pharmaceutical Co., Ltd for the treatment of psychiatric symptoms associated with cerebrovascular diseases, namely depression resulting from stroke, emotional disturbance, and avolition. It was marketed from 1988 to 1998, when it was removed from the market reportedly for lack of effectiveness.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a substituted amphetamine and monoamine releaser similar to MDMA, but with substantially higher activity as a monoaminergic neurotoxin, thought to be due to the unrestrained release of both serotonin and dopamine by a metabolite. It is used as a neurotoxin by neurobiologists to selectively kill serotonergic neurons for research purposes, in the same way that 6-hydroxydopamine is used to kill dopaminergic neurons.

LY-215,840 is an ergoline derivative drug developed by Eli Lilly, which acts as a potent and selective antagonist at the serotonin 5-HT2 and 5-HT7 receptors. It has anti-hypertensive and muscle relaxant effects in animal studies.

Lomerizine (INN) is a diphenylpiperazine class L-type and T-type calcium channel blocker. This drug is currently used clinically for the treatment of migraines, while also being used experimentally for the treatment of glaucoma and optic nerve injury.

IPTBO is a bicyclic phosphate convulsant. It is an extremely potent GABA receptor antagonist that can cause violent convulsions in mice.


















