
Tramadol, sold under the brand name Ultram among others, is an opioid pain medication and a serotonin–norepinephrine reuptake inhibitor (SNRI) used to treat moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen).

Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.

Lefetamine (Santenol) is a drug which is a stimulant and also an analgesic with effects comparable to codeine.

The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(mu)-opioid peptide (MOP) receptors. The prototypical μ-opioid receptor agonist is morphine, the primary psychoactive alkaloid in opium and for which the receptor was named, with mu being the first letter of Morpheus, the compound's namesake in the original Greek. It is an inhibitory G-protein coupled receptor that activates the Gi alpha subunit, inhibiting adenylate cyclase activity, lowering cAMP levels.
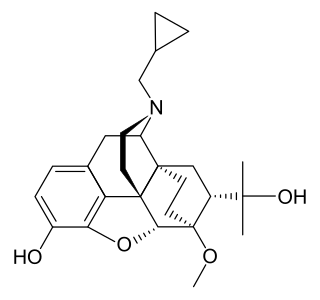
Diprenorphine, also known as diprenorfin, is a non-selective, high-affinity, weak partial agonist of the μ- (MOR), κ- (KOR), and δ-opioid receptor (DOR) which is used in veterinary medicine as an opioid antagonist. It is used to reverse the effects of super-potent opioid analgesics such as etorphine and carfentanil that are used for tranquilizing large animals. The drug is not approved for use in humans.

Desmetramadol, also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with CYP2D6 inactivating mutations.

Oripavine is an opioid and the major metabolite of thebaine. It is the precursor to the semi-synthetic compounds etorphine and buprenorphine. Although this chemical compound has analgesic potency comparable to morphine, it is not used clinically due to severe adverse effects and a low therapeutic index. Being a precursor to a series of extremely strong opioids, oripavine is a controlled substance in some jurisdictions.
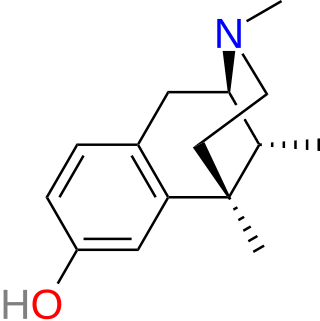
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects, mediated through a mixed agonist–antagonist action at the mu opioid receptor, its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors and/or sigma receptors.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.

Propiram is a partial μ-opioid receptor agonist and weak μ antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Picenadol (LY-97435) is a 4-phenylpiperidine derivative that is an opioid analgesic drug developed by Eli Lilly in the 1970s.

Normorphine is an opiate analogue, the N-demethylated derivative of morphine, that was first described in the 1950s when a large group of N-substituted morphine analogues were characterized for activity. The compound has relatively little opioid activity in its own right, but is a useful intermediate which can be used to produce both opioid antagonists such as nalorphine, and also potent opioid agonists such as N-phenethylnormorphine. with its formation from morphine catalyzed by the liver enzymes CYP3A4 and CYP2C8.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor.
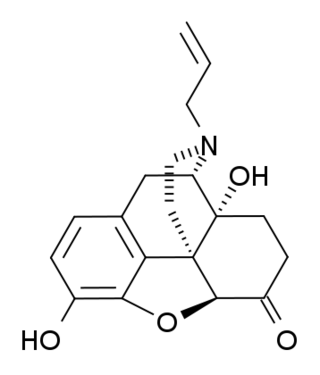
(+)-Naloxone (dextro-naloxone) is a drug which is the opposite enantiomer of the opioid antagonist drug (−)-naloxone. Unlike (−)-naloxone, (+)-naloxone has no significant affinity for opioid receptors, but instead has been discovered to act as a selective antagonist of Toll-like receptor 4. This receptor is involved in immune system responses, and activation of TLR4 induces glial activation and release of inflammatory mediators such as TNF-α and Interleukin-1.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl) piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.
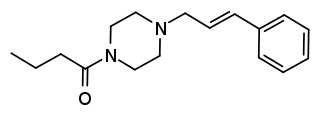
Bucinnazine is an opioid analgesic drug that was widely used in China to treat pain in cancer patients as of 1986. It is one of the most potent compounds among a series of piperazine-amides first synthesized and reported in Japan in the 1970s. Bucinnazine has analgesic potency comparable to that of morphine, but with a relatively higher therapeutic index.

Diphenpipenol is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl)piperazine derivative related to compounds such as MT-45 and AD-1211, but diphenpipenol is the most potent compound in the series, with the more active (S) enantiomer being around 105 times the potency of morphine in animal studies. This makes it a similar strength to fentanyl and its analogues, and consequently diphenpipenol can be expected to pose a significant risk of producing life-threatening respiratory depression, as well as other typical opioid side effects such as sedation, itching, nausea and vomiting.
Peripherally acting μ-opioid receptor antagonists (PAMORAs) are a class of chemical compounds that are used to reverse adverse effects caused by opioids interacting with receptors outside the central nervous system (CNS), mainly those located in the gastrointestinal tract. PAMORAs are designed to specifically inhibit certain opioid receptors in the gastrointestinal tract and with limited ability to cross the blood–brain barrier. Therefore, PAMORAs do not affect the analgesic effects of opioids within the central nervous system.



















