
An analgesic drug, also called simply an analgesic, antalgic, pain reliever, or painkiller, is any member of the group of drugs used for pain management. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects.

Ketoprofen is one of the propionic acid class of nonsteroidal anti-inflammatory drugs (NSAID) with analgesic and antipyretic effects. It acts by inhibiting the body's production of prostaglandin.

Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.
An antipyretic is a substance that reduces fever. Antipyretics cause the hypothalamus to override a prostaglandin-induced increase in temperature. The body then works to lower the temperature, which results in a reduction in fever.

Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used to relieve pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arteriosus in a premature baby. It can be taken orally or intravenously. It typically begins working within an hour.
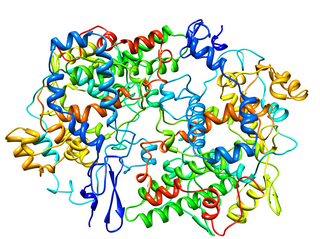
Cyclooxygenase (COX), officially known as prostaglandin-endoperoxide synthase (PTGS), is an enzyme that is responsible for biosynthesis of prostanoids, including thromboxane and prostaglandins such as prostacyclin, from arachidonic acid. A member of the animal-type heme peroxidase family, it is also known as prostaglandin G/H synthase. The specific reaction catalyzed is the conversion from arachidonic acid to prostaglandin H2 via a short-living prostaglandin G2 intermediate.
ATC code M02Topical products for joint and muscular pain is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup M02 is part of the anatomical group M Musculo-skeletal system.

Alfuzosin, sold under the brand name Uroxatral among others, is a medication of the α1 blocker class. It is used to treat benign prostatic hyperplasia (BPH).
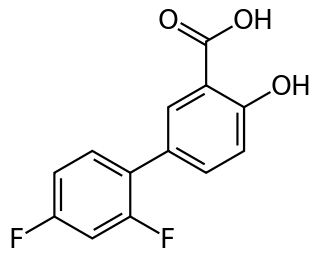
Diflunisal is a salicylic acid derivative with analgesic and anti-inflammatory activity. It was developed by Merck Sharp & Dohme in 1971, as MK647, after showing promise in a research project studying more potent chemical analogs of aspirin. It was first sold under the brand name Dolobid, marketed by Merck & Co., but generic versions are now widely available. It is classed as a nonsteroidal anti-inflammatory drug (NSAID) and is available in 250 mg and 500 mg tablets.

Flurbiprofen is a member of the phenylalkanoic acid derivative family of nonsteroidal anti-inflammatory drugs (NSAIDs). It is primarily indicated as a pre-operative anti-miotic as well as orally for arthritis or dental pain. Side effects are analogous to those of ibuprofen.

Sulindac is a nonsteroidal anti-inflammatory drug (NSAID) of the arylalkanoic acid class that is marketed as Clinoril. Imbaral is another name for this drug. Its name is derived from sul(finyl)+ ind(ene)+ ac(etic acid) It was patented in 1969 and approved for medical use in 1976.

Benzydamine, available as the hydrochloride salt, is a locally acting nonsteroidal anti-inflammatory drug (NSAID) with local anaesthetic and analgesic properties for pain relief and anti-inflammatory treatment of inflammatory conditions of the mouth and throat. It falls under class of chemicals known as indazole.

Tarenflurbil, Flurizan or R-flurbiprofen, is a single enantiomer of the racemate NSAID flurbiprofen. For several years, research and trials for the drug were conducted by Myriad Genetics, to investigate its potential as a treatment for Alzheimer's disease; that investigation concluded in June 2008 when the company announced it would discontinue development of the compound.

Dexketoprofen is a nonsteroidal anti-inflammatory drug (NSAID). It is manufactured by Menarini, under the tradename Keral. It is available in the UK, as dexketoprofen trometamol, as a prescription-only drug and in Latin America as Enantyum, produced by Menarini. Also, in Italy and Spain it is available as an over-the-counter drug (OTC) under the trade name Enandol or Enantyum. In Hungary it is available from a pharmacy as "Ketodex". In Turkey, it is an over the counter medicine under the name "Arveles". In Latvia, Lithuania and Estonia it is available as an OTC under the tradename Dolmen. In Mexico it is available in tablet form as "Stadium" made by Menarini. It is the dextrorotatory stereoisomer of ketoprofen.

Flunoxaprofen, also known as Priaxim, is a chiral nonsteroidal anti-inflammatory drug (NSAID). It is closely related to naproxen, which is also an NSAID. Flunoxaprofen has been shown to significantly improve the symptoms of osteoarthritis and rheumatoid arthritis. The clinical use of flunoxaprofen has ceased due to concerns of potential hepatotoxicity.
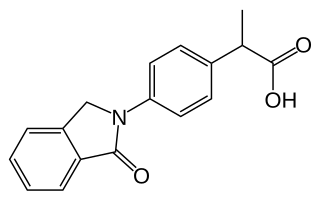
Indoprofen is a nonsteroidal anti-inflammatory drug (NSAID). It was withdrawn worldwide in the 1980s after postmarketing reports of severe gastrointestinal bleeding.

Otenaproxesul is a analgesic and anti-inflammatory drug being developed by Antibe Therapeutics. An NSAID structurally derived from naproxen, in 2016 it received approval to commence phase II clinical trials as a treatment for osteoarthritis after completing phase I clinical trials in 2015. In 2018, the drug completed trials for gastrointestinal safety, and in 2020 completed phase IIb trials on efficacy of pain reduction. Initial phase III clinical trials in 2021 failed to meet the necessary criteria to advance to the next phase.
Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule.

Glucametacin is a non-steroidal anti-inflammatory drug used for the treatment of mild or moderate pain associated with rheumatoid arthritis, osteoarthritis, and other rheumatological disorders. It has analgesic and anti-inflammatory effects.

The profens are a class of nonsteroidal anti-inflammatory drugs. Profens are also known as 2-arylpropionic acids to reflect their chemical structure. The most common example of a profen is ibuprofen, which has been sold under the brand name Profen among others.


















