Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine to the adenosine A1 receptor, which enhances release of the neurotransmitter acetylcholine. Caffeine has a three-dimensional structure similar to that of adenosine, which allows it to bind and block its receptors. Caffeine also increases cyclic AMP levels through nonselective inhibition of phosphodiesterase.

Fluvoxamine, sold under the brand name Luvox among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is primarily used to treat major depressive disorder and obsessive–compulsive disorder (OCD), but is also used to treat anxiety disorders such as panic disorder, social anxiety disorder, and post-traumatic stress disorder.

Alprazolam, sold under the brand name Xanax, among others, is a fast-acting, potent tranquilizer of medium duration in the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It is most commonly used in management of anxiety disorders, specifically panic disorder or generalized anxiety disorder (GAD). Other uses include the treatment of chemotherapy-induced nausea, together with other treatments. GAD improvement occurs generally within a week. Alprazolam is generally taken by mouth.

Clonidine, sold under the brand name Catapres among others, is an α2-adrenergic agonist medication used to treat high blood pressure, ADHD, drug withdrawal, menopausal flushing, diarrhea, spasticity, and certain pain conditions. It is used by mouth, by injection, or as a skin patch. Onset of action is typically within an hour with the effects on blood pressure lasting for up to eight hours.

Triazolam, sold under the brand name Halcion among others, is a central nervous system (CNS) depressant tranquilizer of the triazolobenzodiazepine (TBZD) class, which are benzodiazepine (BZD) derivatives. It possesses pharmacological properties similar to those of other benzodiazepines, but it is generally only used as a sedative to treat severe insomnia. In addition to the hypnotic properties, triazolam's amnesic, anxiolytic, sedative, anticonvulsant, and muscle relaxant properties are pronounced as well.
Ergotamine, sold under the brand names Cafergot and Ergomar among others, is an ergopeptine and part of the ergot family of alkaloids; it is structurally and biochemically closely related to ergoline. It possesses structural similarity to several neurotransmitters, and has biological activity as a vasoconstrictor.

Zopiclone, sold under the brand name Imovane among others, is a nonbenzodiazepine used to treat difficulty sleeping. Zopiclone is molecularly distinct from benzodiazepine drugs and is classed as a cyclopyrrolone. However, zopiclone increases the normal transmission of the neurotransmitter gamma-aminobutyric acid (GABA) in the central nervous system, via modulating GABAA receptors similarly to the way benzodiazepine drugs do.

Bromazepam, sold under many brand names, is a benzodiazepine. It is mainly an anti-anxiety agent with similar side effects to diazepam (Valium). In addition to being used to treat anxiety or panic states, bromazepam may be used as a premedicant prior to minor surgery. Bromazepam typically comes in doses of 3 mg and 6 mg tablets.

Baclofen, sold under the brand name Lioresal among others, is a medication used to treat muscle spasticity such as from a spinal cord injury or multiple sclerosis. It may also be used for hiccups and muscle spasms near the end of life. It is taken by mouth or by delivery into the spinal canal.
Haloperidol decanoate, sold under the brand name Haldol Decanoate among others, is a typical antipsychotic which is used in the treatment of schizophrenia. It is administered by injection into muscle at a dose of 100 to 200 mg once every 4 weeks or monthly. The dorsogluteal site is recommended. A 3.75-cm (1.5-inch), 21-gauge needle is generally used, but obese individuals may require a 6.5-cm (2.5-inch) needle to ensure that the drug is indeed injected intramuscularly and not subcutaneously. Haloperidol decanoate is provided in the form of 50 or 100 mg/mL oil solution of sesame oil and benzyl alcohol in ampoules or pre-filled syringes. Its elimination half-life after multiple doses is 21 days. The medication is marketed in many countries throughout the world.

Cinnarizine is an antihistamine and calcium channel blocker of the diphenylmethylpiperazine group. It is prescribed for nausea and vomiting due to motion sickness or other sources such as chemotherapy, vertigo, or Ménière's disease.
Bisoprolol, sold under the brand name Zebeta among others, is a beta blocker medication used for heart diseases. This includes tachyarrhythmias, high blood pressure, chest pain from not enough blood flow to the heart, and heart failure. It is taken by mouth.

Paraxanthine, or 1,7-dimethylxanthine, is a dimethyl derivative of xanthine, structurally related to caffeine.
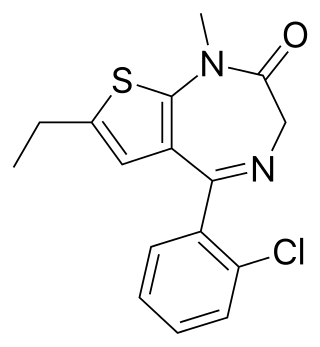
Clotiazepam is a thienodiazepine drug which is a benzodiazepine analog. The clotiazepam molecule differs from benzodiazepines in that the benzene ring has been replaced by a thiophene ring. It possesses anxiolytic, skeletal muscle relaxant, anticonvulsant, sedative properties. Stage 2 NREM sleep is significantly increased by clotiazepam.
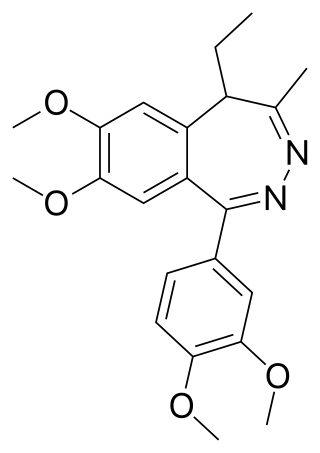
Tofisopam is an anxiolytic that is marketed in several European countries. Chemically, it is a 2,3-benzodiazepine. Unlike other anxiolytic benzodiazepines however, tofisopam does not have anticonvulsant, sedative, skeletal muscle relaxant, motor skill-impairing or amnestic properties. While it may not be an anticonvulsant in and of itself, it has been shown to enhance the anticonvulsant action of classical 1,4-benzodiazepines and muscimol, but not sodium valproate, carbamazepine, phenobarbital, or phenytoin. Tofisopam is indicated for the treatment of anxiety and alcohol withdrawal, and is prescribed in a dosage of 50–300 mg per day divided into three doses. Peak plasma levels are attained two hours after an oral dose. Tofisopam is not reported as causing dependence to the same extent as other benzodiazepines, but is still recommended to be prescribed for a maximum of 12 weeks.
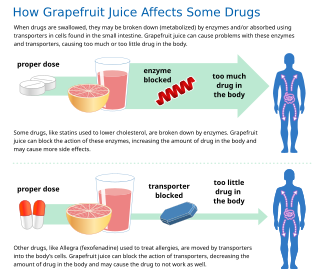
Some fruit juices and fruits can interact with numerous drugs, in many cases causing adverse effects. The effect is most studied with grapefruit and grapefruit juice, but similar effects have been observed with certain other citrus fruits.
Gliclazide, sold under the brand name Diamicron among others, is a sulfonylurea type of anti-diabetic medication, used to treat type 2 diabetes. It is used when dietary changes, exercise, and weight loss are not enough. It is taken by mouth.

Delorazepam, also known as chlordesmethyldiazepam and nordiclazepam, is a drug which is a benzodiazepine and a derivative of desmethyldiazepam. It is marketed in Italy, where it is available under the trade name EN and Dadumir. Delorazepam (chlordesmethyldiazepam) is also an active metabolite of the benzodiazepine drugs diclazepam and cloxazolam. Adverse effects may include hangover type effects, drowsiness, behavioural impairments and short-term memory impairments. Similar to other benzodiazepines delorazepam has anxiolytic, skeletal muscle relaxant, hypnotic and anticonvulsant properties.

Ulimorelin is a drug with a modified cyclic peptide structure which acts as a selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR-1a).. Unlike many related drugs, ulimorelin has little or no effect on growth hormone (GH) release in rats. However, like ghrelin and other ghrelin agonists, ulimorelin does stimulate GH release with concomitant increases in insulin-like growth factor 1 (IGF-1) in humans. It has been researched for enhancing gastrointestinal motility, especially in gastroparesis and in aiding recovery of bowel function following gastrointestinal surgery, where opioid analgesic drugs used for post-operative pain relief may worsen existing constipation. While ulimorelin has been shown to increase both upper and lower gastrointestinal motility in rats, and showed promising results initially in humans, it failed in pivotal clinical trials in post operative ileus.

GSK2881078 is a drug which acts as a selective androgen receptor modulator (SARM). It was developed for the prevention of muscle wasting and sarcopenia in elderly people.