In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula CH3. In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond, it can be found on its own in any of three forms: methanide anion, methylium cation or methyl radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.
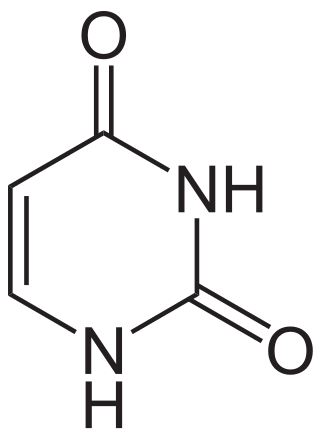
Uracil is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine (T). Uracil is a demethylated form of thymine.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2.

Thujone is a ketone and a monoterpene that occurs predominantly in two diastereomeric (epimeric) forms: (−)-α-thujone and (+)-β-thujone.
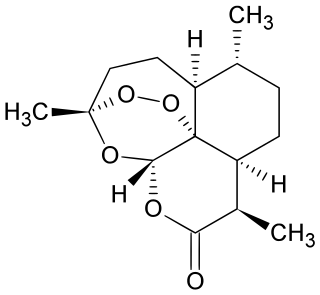
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua, sweet wormwood, a herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.
In the physical sciences, a partition coefficient (P) or distribution coefficient (D) is the ratio of concentrations of a compound in a mixture of two immiscible solvents at equilibrium. This ratio is therefore a comparison of the solubilities of the solute in these two liquids. The partition coefficient generally refers to the concentration ratio of un-ionized species of compound, whereas the distribution coefficient refers to the concentration ratio of all species of the compound.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.
Volatile organic compounds (VOCs) are organic compounds that have a high vapor pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a trait known as volatility.

Streptomyces is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin.

An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated.
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.
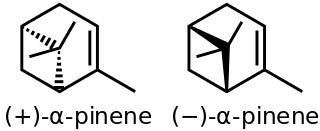
α-Pinene is an organic compound of the terpene class, one of two isomers of pinene. It is an alkene and it contains a reactive four-membered ring. It is found in the oils of many species of many coniferous trees, notably the pine. It is also found in the essential oil of rosemary and Satureja myrtifolia. Both enantiomers are known in nature; (1S,5S)- or (−)-α-pinene is more common in European pines, whereas the (1R,5R)- or (+)-α-isomer is more common in North America. The racemic mixture is present in some oils such as eucalyptus oil and orange peel oil.
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

Prodigiosin is the red dyestuff produced by many strains of the bacterium Serratia marcescens, as well as other Gram-negative, gamma proteobacteria such as Vibrio psychroerythrus and Hahella chejuensis. It is responsible for the pink tint occasionally found in grime that accumulates on porcelain surfaces such as bathtubs, sinks, and toilet bowls. It is in the prodiginines family of compounds which are produced in some Gram-negative gamma proteobacteria, as well as select Gram-positive Actinobacteria. The name prodigiosin is derived from prodigious.

6-Phosphogluconolactonase (EC 3.1.1.31, 6PGL, PGLS, systematic name 6-phospho-D-glucono-1,5-lactone lactonohydrolase) is a cytosolic enzyme found in all organisms that catalyzes the hydrolysis of 6-phosphogluconolactone to 6-phosphogluconic acid in the oxidative phase of the pentose phosphate pathway:

Ganaplacide is a drug in development by Novartis for the purpose of treating malaria. It belongs to the class of the imidazolopiperazines. It has shown activity against the Plasmodium falciparum and Plasmodium vivax forms of the malaria parasite.

Ferroquine is a synthetic compound related to chloroquine which acts as an antimalarial, and shows good activity against chloroquine-resistant strains. It contains an organometallic ferrocene ring which is unusual in pharmaceuticals, and while it was first reported in 1997, it has progressed slowly through clinical trials, with results from Phase II trials showing reasonable safety and efficacy, and further trials ongoing.












