
Ayahuasca is a South American psychoactive beverage, traditionally used by Indigenous cultures and folk healers in the Amazon and Orinoco basins for spiritual ceremonies, divination, and healing a variety of psychosomatic complaints.

N,N-Dimethyltryptamine is a substituted tryptamine that occurs in many plants and animals, including humans, and which is both a derivative and a structural analog of tryptamine. DMT is used as a psychedelic drug and prepared by various cultures for ritual purposes as an entheogen.
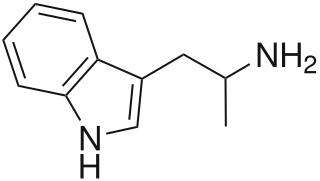
α-Methyltryptamine is a psychedelic, stimulant, and entactogen drug of the tryptamine family. It was originally developed as an antidepressant at Upjohn in the 1960s, and was used briefly as an antidepressant in the Soviet Union under the brand name Indopan or Indopane before being discontinued.

Banisteriopsis caapi, also known as, caapi, soul vine, yagé (yage), or ayahuasca, the latter of which also refers to the psychedelic decoction made with the vine and a plant source of dimethyltryptamine, is a South American liana of the family Malpighiaceae. It is commonly used as an ingredient of ayahuasca, a decoction with a long history of its entheogenic use and holds status as a "plant teacher" among the Indigenous peoples of the Amazon rainforest.
5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine), also known as O-methylbufotenin or mebufotenin, is a naturally occurring psychedelic of the tryptamine family. It is found in a wide variety of plant species, and is also secreted by the glands of at least one toad species, the Colorado River toad. It may occur naturally in humans as well. Like its close relatives dimethyltryptamine (DMT) and bufotenin (5-HO-DMT), it has been used as an entheogen in South America. Slang terms include Five-methoxy, the power, bufo, and toad venom.

β-Carboline (9H-pyrido[3,4-b]indole) represents the basic chemical structure for more than one hundred alkaloids and synthetic compounds. The effects of these substances depend on their respective substituent. Natural β-carbolines primarily influence brain functions but can also exhibit antioxidant effects. Synthetically designed β-carboline derivatives have recently been shown to have neuroprotective, cognitive enhancing and anti-cancer properties.
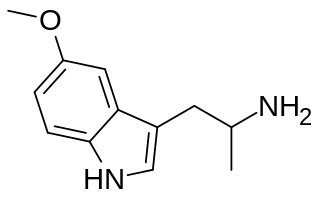
5-MeO-αMT, or 5-methoxy-α-methyltryptamine, also known as α,O-dimethylserotonin (Alpha-O), is a serotonergic psychedelic of the tryptamine family. It is a derivative of α-methyltryptamine (αMT) and an analogue of 5-MeO-DMT.

Harmala alkaloids are several alkaloids that act as monoamine oxidase inhibitors (MAOIs). These alkaloids are found in the seeds of Peganum harmala, as well as Banisteriopsis caapi (ayahuasca), leaves of tobacco and coffee beans. The alkaloids include harmine, harmaline, harmalol, and their derivatives, which have similar chemical structures, hence the name "harmala alkaloids". These alkaloids are of interest for their use in Amazonian shamanism, where they are derived from other plants. Harmine, once known as telepathine and banisterine, is a naturally occurring beta-carboline alkaloid that is structurally related to harmaline, and also found in the vine Banisteriopsis caapi. Tetrahydroharmine is also found in B. caapi and P. harmala. Dr. Alexander Shulgin has suggested that harmine may be a breakdown product of harmaline. Harmine and harmaline are reversible inhibitors of monoamine oxidase A (RIMAs). They can stimulate the central nervous system by inhibiting the metabolism of monoamine compounds such as serotonin and norepinephrine.

DET, also known under its chemical name N,N-diethyltryptamine and as T-9, is a psychedelic drug closely related to DMT and 4-HO-DET. However, despite its structural similarity to DMT, its activity is induced by an oral dose of around 50–100 mg, without the aid of MAO inhibitors, and the effects last for about 2–4 hours.
Harmine is a beta-carboline and a harmala alkaloid. It occurs in a number of different plants, most notably the Syrian rue and Banisteriopsis caapi. Harmine reversibly inhibits monoamine oxidase A (MAO-A), an enzyme which breaks down monoamines, making it a Reversible inhibitor of monoamine oxidase A (RIMA). Harmine does not inhibit MAO-B. Harmine is also known as banisterin, banisterine, telopathin, telepathine, leucoharmine and yagin, yageine.

Harmaline is a fluorescent indole alkaloid from the group of harmala alkaloids and beta-carbolines. It is the partly hydrogenated form of harmine.
Pharmahuasca is a pharmaceutical version of the entheogenic brew ayahuasca. Traditional ayahuasca is made by brewing the MAOI-containing Banisteriopsis caapi vine with a DMT-containing plant, such as Psychotria viridis. Pharmahuasca refers to a similar combination that uses a pharmaceutical MAOI instead of a plant.

Tetrahydroharmol is a bioactive beta-carboline harmala alkaloid. It acts as a reversible inhibitor of monoamine oxidase A.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Tryptoline, also known as tetrahydro-β-carboline and tetrahydronorharmane, is a natural organic derivative of beta-carboline. It is an alkaloid chemically related to tryptamines. Derivatives of tryptoline have a variety of pharmacological properties and are known collectively as tryptolines.
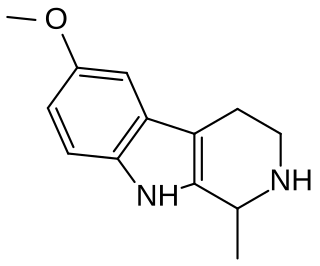
6-MeO-THH, or 6-methoxy-1,2,3,4-tetrahydroharman, is a β-carboline derivative and a structural isomer of tetrahydroharmine (7-MeO-THH). 6-MeO-THH is mentioned in Alexander Shulgin's book TiHKAL, stating that 6-MeO-THH is very similar to the other carbolines. Limited testing suggests that it possesses mild psychoactive effects at 1.5 mg/kg and is said to be about one-third as potent as 6-methoxyharmalan. It has been isolated from certain plants of the Virola family.

3-Fluoroamphetamine is a stimulant drug from the amphetamine family which acts as a monoamine releaser with similar potency to methamphetamine but more selectivity for dopamine and norepinephrine release over serotonin. It is self-administered by mice to a similar extent to related drugs such as 4-fluoroamphetamine and 3-methylamphetamine.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

Changa is a blend of N,N-Dimethyltryptamine (DMT) mixed with a monoamine oxidase inhibitor (MAOI). The addition of MAOIs extends the DMT experience in duration and intensity when compared with smoking DMT freebase alone. Typically, extracts from DMT-containing plants are combined with a blend of different MAOI-containing herbs, such as the ayahuasca vine, and/or leaf or harmala alkaloids from Peganum harmala to create a mix that is 25 to 50% DMT.

















