
Estrone (E1), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estriol. Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads, though they can also be formed from adrenal androgens in adipose tissue. Relative to estradiol, both estrone and estriol have far weaker activity as estrogens. Estrone can be converted into estradiol, and serves mainly as a precursor or metabolic intermediate of estradiol. It is both a precursor and metabolite of estradiol.
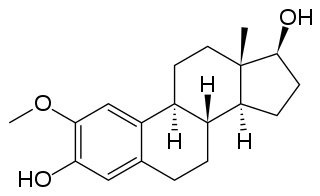
2-Methoxyestradiol is a natural metabolite of estradiol and 2-hydroxyestradiol (2-OHE2). It is specifically the 2-methyl ether of 2-hydroxyestradiol. 2-Methoxyestradiol prevents the formation of new blood vessels that tumors need in order to grow (angiogenesis), hence it is an angiogenesis inhibitor. It also acts as a vasodilator and induces apoptosis in some cancer cell lines. 2-Methoxyestradiol is derived from estradiol, although it interacts poorly with the estrogen receptors. However, it retains activity as a high-affinity agonist of the G protein-coupled estrogen receptor (GPER).

Normetanephrine, also called normetadrenaline, is a metabolite of norepinephrine created by action of catechol-O-methyl transferase on norepinephrine. It is excreted in the urine and found in certain tissues. It is a marker for catecholamine-secreting tumors such as pheochromocytoma.

2-Hydroxyestradiol (2-OHE2), also known as estra-1,3,5(10)-triene-2,3,17β-triol, is an endogenous steroid, catechol estrogen, and metabolite of estradiol, as well as a positional isomer of estriol.

16α-Hydroxyestrone (16α-OH-E1), or hydroxyestrone, also known as estra-1,3,5(10)-triene-3,16α-diol-17-one, is an endogenous steroidal estrogen and a major metabolite of estrone, as well as an intermediate in the biosynthesis of estriol. It is a potent estrogen similarly to estrone, and it has been suggested that the ratio of 16α-hydroxyestrone to 2-hydroxyestrone, the latter being much less estrogenic in comparison and even antiestrogenic in the presence of more potent estrogens like estradiol, may be involved in the pathophysiology of breast cancer. Conversely, 16α-hydroxyestrone may help to protect against osteoporosis.

A catechol estrogen is a steroidal estrogen that contains catechol (1,2-dihydroxybenzene) within its structure. The catechol estrogens are endogenous metabolites of estradiol and estrone and include the following compounds:

Estriol glucuronide (E3G), or oestriol glucuronide, also known as estriol monoglucuronide, as well as estriol 16α-β-D-glucosiduronic acid, is a natural, steroidal estrogen and the glucuronic acid conjugate of estriol. It occurs in high concentrations in the urine of pregnant women as a reversibly formed metabolite of estriol. Estriol glucuronide is a prodrug of estriol, and was the major component of Progynon and Emmenin, estrogenic products manufactured from the urine of pregnant women that were introduced in the 1920s and 1930s and were the first orally active estrogens. Emmenin was succeeded by Premarin, which is sourced from the urine of pregnant mares and was introduced in 1941. Premarin replaced Emmenin due to the fact that it was easier and less expensive to produce.

Estradiol glucuronide, or estradiol 17β-D-glucuronide, is a conjugated metabolite of estradiol. It is formed from estradiol in the liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys. It has much higher water solubility than does estradiol. Glucuronides are the most abundant estrogen conjugates.
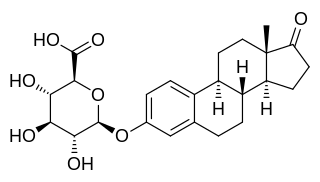
Estrone glucuronide, or estrone-3-D-glucuronide, is a conjugated metabolite of estrone. It is formed from estrone in the liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys. It has much higher water solubility than does estrone. Glucuronides are the most abundant estrogen conjugates and estrone glucuronide is the dominant metabolite of estradiol.

Estriol sulfate glucuronide, or estriol 3-sulfate 16α-glucuronide, is an endogenous, naturally occurring diconjugated metabolite of estriol. It is generated in the liver from estriol sulfate by UDP-glucuronyltransferase and is eventually excreted in the urine by the kidneys. It occurs in high concentrations during pregnancy along with estriol sulfate and estriol glucuronide, and was a component of the early pharmaceutical estrogens Progynon and Emmenin.

2-Hydroxyestrone (2-OHE1), also known as estra-1,3,5(10)-trien-2,3-diol-17-one, is an endogenous, naturally occurring catechol estrogen and a major metabolite of estrone and estradiol. It is formed irreversibly from estrone in the liver and to a lesser extent in other tissues via 2-hydroxylation mediated by cytochrome P450 enzymes, mainly the CYP3A and CYP1A subfamilies. 2-OHE1 is the most abundant catechol estrogen in the body.

4-Hydroxyestradiol (4-OHE2), also known as estra-1,3,5(10)-triene-3,4,17β-triol, is an endogenous, naturally occurring catechol estrogen and a minor metabolite of estradiol. It is estrogenic, similarly to many other hydroxylated estrogen metabolites such as 2-hydroxyestradiol, 16α-hydroxyestrone, estriol (16α-hydroxyestradiol), and 4-hydroxyestrone but unlike 2-hydroxyestrone.
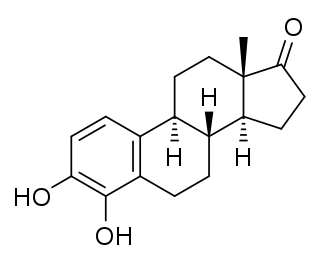
4-Hydroxyestrone (4-OHE1), also known as estra-1,3,5(10)-triene-3,4-diol-17-one, is an endogenous, naturally occurring catechol estrogen and a minor metabolite of estrone and estradiol. It is estrogenic, similarly to many other hydroxylated estrogen metabolites such as 2-hydroxyestradiol, 16α-hydroxyestrone, estriol (16α-hydroxyestradiol), and 4-hydroxyestradiol but unlike 2-hydroxyestrone.
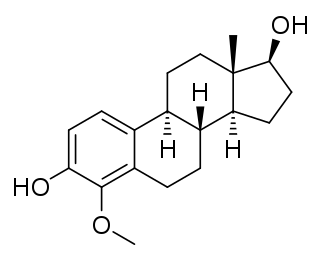
4-Methoxyestradiol (4-ME2) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estradiol that is formed by catechol O-methyltransferase via the intermediate 4-hydroxyestradiol. It has estrogenic activity similarly to estrone and 4-hydroxyestrone.

4-Methoxyestrone (4-ME1) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estrone that is formed by catechol O-methyltransferase via the intermediate 4-hydroxyestrone. It has estrogenic activity similarly to estrone and 4-hydroxyestrone.

Estriol 3-glucuronide, or oestriol 3-glucuronide, also known as estriol 3-β-D-glucosiduronic acid, is a natural, steroidal estrogen and a glucuronic acid conjugate of estriol. It is found in the urine of women as a reversibly formed metabolite of estriol. The positional isomer of estriol 3-glucuronide, estriol 16α-glucuronide, also occurs as an endogenous metabolite of estriol, but to a much greater extent in comparison.

Etiocholanedione, also known as 5β-androstanedione or as etiocholane-3,17-dione, is a naturally occurring etiocholane (5β-androstane) steroid and an endogenous metabolite of androgens like testosterone, dihydrotestosterone, dehydroepiandrosterone (DHEA), and androstenedione. It is the C5 epimer of androstanedione (5α-androstanedione). Although devoid of androgenic activity like other 5β-reduced steroids, etiocholanedione has some biological activity of its own. The compound has been found to possess potent haematopoietic effects in a variety of models. In addition, it has been found to promote weight loss in animals and in a double-blind, placebo-controlled clinical study in humans conducted in 1993. These effects are said to be similar to those of DHEA. Unlike DHEA however, etiocholanedione cannot be metabolized further into steroid hormones like androgens and estrogens.

Estradiol 3-glucuronide (E2-3G), also known as 17β-estradiol 3-(β-D-glucuronide), is a naturally occurring and endogenous estrogen conjugate. It is specifically the C3 glucuronide conjugate of estradiol, the major estrogen in the body. It is formed from estradiol in the liver by UDP-glucuronosyltransferase via attachment of glucuronic acid and is eventually excreted in urine and bile. Similarly to estrogen sulfates like estrone sulfate, estrogen glucuronides have much higher water solubility than do unconjugated estrogens like estradiol.

16-Ketoestradiol is an endogenous estrogen related to 16-ketoestrone. 16-Ketoestrone is a very weak estrogen with only 1/1000 the estrogenic potency of estradiol in the uterus. It is a so-called "short-acting" or "impeded" estrogen, similarly to estriol and dimethylstilbestrol.

4-Methoxyestriol (4-MeO-E3) is an endogenous estrogen metabolite. It is the 4-methyl ether of 4-hydroxyestriol and a metabolite of estriol and 4-hydroxyestriol. 4-Methoxyestriol has very low affinities for the estrogen receptors. Its relative binding affinities (RBAs) for estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) are both about 1% of those of estradiol. For comparison, estriol had RBAs of 11% and 35%, respectively.



















