
Estradiol acetate (EA), sold under the brand names Femtrace, Femring, and Menoring, is an estrogen medication which is used in hormone therapy for the treatment of menopausal symptoms in women. It is taken by mouth once daily or given as a vaginal ring once every three months.

Estradiol valerate (EV), sold for use by mouth under the brand name Progynova and for use by injection under the brand names Delestrogen and Progynon Depot among others, is an estrogen medication. It is used in hormone therapy for menopausal symptoms and low estrogen levels, hormone therapy for transgender people, and in hormonal birth control. It is also used in the treatment of prostate cancer. The medication is taken by mouth or by injection into muscle or fat once every 1 to 4 weeks.

Estrone sulfate, also known as E1S, E1SO4 and estrone 3-sulfate, is a natural, endogenous steroid and an estrogen ester and conjugate.

Estradiol benzoate (EB), sold under the brand name Progynon-B among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for transgender women, and in the treatment of gynecological disorders. It is also used in the treatment of prostate cancer in men. Estradiol benzoate is used in veterinary medicine as well. When used clinically, the medication is given by injection into muscle usually two to three times per week.

Estradiol cypionate (EC), sold under the brand name Depo-Estradiol among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for trans women, and in hormonal birth control for women. It is given by injection into muscle once every 1 to 4 weeks.
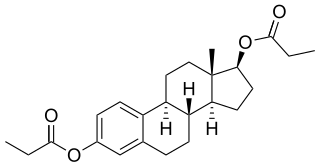
Estradiol dipropionate (EDP), sold under the brand names Agofollin, Di-Ovocylin, and Progynon DP among others, is an estrogen medication which has been used in hormone therapy for menopausal symptoms and low estrogen levels in women and in the treatment of gynecological disorders. It has also been used in feminizing hormone therapy for transgender women and in the treatment of prostate cancer in men. Although widely used in the past, estradiol dipropionate has largely been discontinued and is mostly no longer available today. It appears to remain in use only in Japan, Macedonia, and Australia. Estradiol dipropionate is given by injection into muscle at intervals ranging from once or twice a week to once every week and a half to two weeks.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.
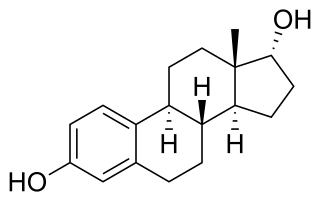
17α-Estradiol is a minor and weak endogenous steroidal estrogen that is related to 17β-estradiol. It is the C17 epimer of estradiol. It has approximately 100-fold lower estrogenic potency than 17β-estradiol. The compound shows preferential affinity for the ERα over the ERβ. Although 17α-estradiol is far weaker than 17β-estradiol as an agonist of the nuclear estrogen receptors, it has been found to bind to and activate the brain-expressed ER-X with a greater potency than that of 17β-estradiol, suggesting that it may be the predominant endogenous ligand for the receptor.

Estradiol hexahydrobenzoate (EHHB), sold under a number of brand names including Benzo-Ginoestril A.P., BenzoGynoestryl Retard, Ginestryl-15-Depot, Menodin, and Tardoginestryl, is an estrogen medication which was previously used for indications such as menopausal hormone therapy and gynecological disorders. EHHB is given by injection into muscle at regular intervals, for instance once every few weeks.
Estradiol palmitate, or estradiol monopalmitate, also known as estradiol 17β-hexadecanoate, is a naturally occurring steroidal estrogen and an estrogen ester – specifically, the C17β palmitate ester of estradiol. It occurs in the body as a very long-lasting metabolite and prohormone of estradiol. The compound has no affinity for the estrogen receptor, requiring transformation into estradiol for its estrogenic activity. In addition to its endogenous role, estradiol palmitate was formerly used as a fattening agent in chickens under the brand name Esmopal.
Estradiol stearate (E2-17-St), also known as estradiol octadecanoate and sold under the brand name Depofollan, is a naturally occurring estrogen and an estrogen ester – specifically, the C17β stearate ester of estradiol. It occurs in the body as a very long-lasting metabolite and prohormone of estradiol. The compound is one of the components that collectively constitute lipoidal estradiol, another of which is estradiol palmitate. It is extremely lipophilic and hydrophobic. Estradiol stearate has no affinity for the estrogen receptor, requiring transformation into estradiol via esterases for its estrogenic activity. The compound does not bind to sex hormone-binding globulin or α-fetoprotein, instead being transported by lipoproteins such as high-density lipoprotein and low-density lipoprotein.

Estradiol dienanthate (EDE), sold under the brand names Climacteron among others, is a long-acting estrogen medication which was previously used in menopausal hormone therapy for women and to suppress lactation in women. It was formulated in combination with estradiol benzoate (EB), a short-acting estrogen, and testosterone enanthate benzilic acid hydrazone (TEBH), a long-acting androgen/anabolic steroid. EDE has not been made available for medical use alone. The medication, in combination with EB and TEBH, was given by injection into muscle once or at regular intervals, for instance once every 6 weeks.

A steroid ester is an ester of a steroid. They include androgen esters, estrogen esters, progestogen esters, and corticosteroid esters. Steroid esters may be naturally occurring/endogenous like DHEA sulfate or synthetic like estradiol valerate. Esterification is useful because it is often able to render the parent steroid into a prodrug of itself with altered chemical properties such as improved metabolic stability, water solubility, and/or lipophilicity. This, in turn, can enhance pharmacokinetics, for instance by improving the steroid's bioavailability and/or conferring depot activity and hence an extended duration with intramuscular or subcutaneous injection.

Estradiol mustard, also known as estradiol 3,17β-bis(4- phenyl)acetate, is a semisynthetic, steroidal estrogen and cytostatic antineoplastic agent and a phenylacetic acid nitrogen mustard-coupled estrogen ester that was never marketed. It is selectively distributed into estrogen receptor (ER)-positive tissues such as ER-expressing tumors like those seen in breast and prostate cancers. For this reason, estradiol mustard and other cytostatic-linked estrogens like estramustine phosphate have reduced toxicity relative to non-linked nitrogen mustard cytostatic antineoplastic agents. However, they may stimulate breast tumor growth due to their inherent estrogenic activity and are said to be devoid of major therapeutic efficacy in breast cancer, although estramustine phosphate has been approved for and is used in the treatment of prostate cancer.
Testosterone stearate, also known as testosterone octadecanoate, testosterone 17β-stearate, and androst-4-en-17β-ol-3-one 17β-stearate, is an injected anabolic-androgenic steroid (AAS) and an androgen ester – specifically, the C17β stearate (octadecanoate) ester of testosterone – which was never marketed. It is a prodrug of testosterone and, when administered via intramuscular injection, is associated with a long-lasting depot effect and extended duration of action. Testosterone stearate may occur naturally in the body.

Estradiol glucuronide, or estradiol 17β-D-glucuronide, is a conjugated metabolite of estradiol. It is formed from estradiol in the liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys. It has much higher water solubility than does estradiol. Glucuronides are the most abundant estrogen conjugates.

An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.

Estriol (E3), sold under the brand name Ovestin among others, is an estrogen medication and naturally occurring steroid hormone which is used in menopausal hormone therapy. It is also used in veterinary medicine as Incurin to treat urinary incontinence due to estrogen deficiency in dogs. The medication is taken by mouth in the form of tablets, as a cream that is applied to the skin, as a cream or pessary that is applied in the vagina, and by injection into muscle.

Estrone sulfate (E1S) is an estrogen medication and naturally occurring steroid hormone. It is used in menopausal hormone therapy among other indications. As the sodium salt, it is the major estrogen component of conjugated estrogens (Premarin) and esterified estrogens. In addition, E1S is used on its own as the piperazine salt estropipate. The compound also occurs as a major and important metabolite of estradiol and estrone. E1S is most commonly taken by mouth, but in the form of Premarin can also be taken by parenteral routes such as transdermal, vaginal, and injection.

The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.













