
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues. However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors, and acting as a neurosteroid and modulator of neurotrophic factor receptors.

Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the major progestogen in the body. Progesterone has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.

Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.
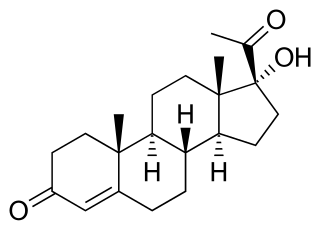
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Hydroxyprogesterone caproate, sold under the brand names Proluton and Makena among others, is a medication used to reduce the risk of preterm birth in women pregnant with one baby who have a history of spontaneous preterm birth. In March 2023, the manufacturer, Covis Pharma, agreed to withdraw the drug from the US market. The approvals of Makena and its generics were withdrawn by the US Food and Drug Administration (FDA) in April 2023.

5α-Dihydroprogesterone is an endogenous progestogen and neurosteroid that is synthesized from progesterone. It is also an intermediate in the synthesis of allopregnanolone and isopregnanolone from progesterone.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Promegestone, sold under the brand name Surgestone, is a progestin medication which is used in menopausal hormone therapy and in the treatment of gynecological disorders. It is taken by mouth.

Trimegestone, sold under the brand names Ondeva and Totelle among others, is a progestin medication which is used in menopausal hormone therapy and in the prevention of postmenopausal osteoporosis. It was also under development for use in birth control pills to prevent pregnancy, but ultimately was not marketed for this purpose. The medication is available alone or in combination with an estrogen. It is taken by mouth.
Membrane progesterone receptors (mPRs) are a group of cell surface receptors and membrane steroid receptors belonging to the progestin and adipoQ receptor (PAQR) family which bind the endogenous progestogen and neurosteroid progesterone, as well as the neurosteroid allopregnanolone. Unlike the progesterone receptor (PR), a nuclear receptor which mediates its effects via genomic mechanisms, mPRs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades. The mPRs mediate important physiological functions in male and female reproductive tracts, liver, neuroendocrine tissues, and the immune system as well as in breast and ovarian cancer.

20α-Dihydroprogesterone (20α-DHP), also known as 20α-hydroxyprogesterone (20α-OHP), is a naturally occurring, endogenous progestogen. It is a metabolite of progesterone, formed by the 20α-hydroxysteroid dehydrogenases (20α-HSDs) AKR1C1, AKR1C2, and AKR1C3 and the 17β-hydroxysteroid dehydrogenase (17β-HSD) HSD17B1. 20α-DHP can be transformed back into progesterone by 20α-HSDs and by the 17β-HSD HSD17B2. HSD17B2 is expressed in the human endometrium and cervix among other tissues. In animal studies, 20α-DHP has been found to be selectively taken up into and retained in target tissues such as the uterus, brain, and skeletal muscle.

11α-Hydroxyprogesterone (11α-OHP), or 11α-hydroxypregn-4-ene-3,20-dione is an endogenous steroid and metabolite of progesterone. It is a weak antiandrogen, and is devoid of androgenic, estrogenic, and progestogenic activity.

5α-Dihydronorethisterone is a major active metabolite of norethisterone (norethindrone). Norethisterone is a progestin with additional weak androgenic and estrogenic activity. 5α-DHNET is formed from norethisterone by 5α-reductase in the liver and other tissues.

7α-Thioprogesterone is a synthetic, steroidal, and potent antimineralocorticoid (putative) and antiandrogen which was developed by G. D. Searle & Co and was described in the late 1970s and early 1980s but was never developed or introduced for medical use. It is a derivative of progesterone (pregn-4-ene-3,20-dione) with a thio (sulfur) substitution at the C7α position, and is related to the spirolactone group of drugs but lacks a γ-lactone ring.

5α-Dihydroethisterone is an active metabolite of the formerly clinically used but now-discontinued progestin ethisterone and the experimental and never-marketed hormonal antineoplastic agent ethynylandrostanediol (HE-3235). Its formation from its parent drugs is catalyzed by 5α-reductase in tissues that express the enzyme in high amounts like the liver, skin, hair follicles, and prostate gland. 5α-DHET has significant affinity for steroid hormone receptors and may contribute importantly to the activities of its parent drugs.

20β-Dihydroprogesterone (20β-DHP), also known as 20β-hydroxyprogesterone (20β-OHP), is an endogenous metabolite of progesterone which is formed by 20β-hydroxysteroid dehydrogenase (20β-HSD). It is a progestogen similarly to progesterone, with about 20 to 50% of the progestogenic activity of progesterone. It can be converted by 20β-HSD into progesterone in the uterus. The effects of 20β-HSD on the uterus, mammary glands, and in maintaining pregnancy have been studied. The progestogenic activity of 20β-HSD has also been characterized in women.
The pharmacology of progesterone, a progestogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.
The androgen backdoor pathway is a collective name for all metabolic pathways where clinically relevant androgens are synthesized from 21-carbon steroids (pregnanes) by their 5α-reduction, bypassing testosterone and/or androstenedione.

5α-Pregnan-17α-ol-3,20-dione, also known as 17α-hydroxy-dihydroprogesterone (17‐OH-DHP) is an endogenous steroid, a metabolite of 17α-hydroxyprogesterone.
















