Estriol (E3), also spelled oestriol, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estrone. Levels of estriol in women who are not pregnant are almost undetectable. However, during pregnancy, estriol is synthesized in very high quantities by the placenta and is the most produced estrogen in the body by far, although circulating levels of estriol are similar to those of other estrogens due to a relatively high rate of metabolism and excretion. Relative to estradiol, both estriol and estrone have far weaker activity as estrogens.
2-Methoxyestradiol is a natural metabolite of estradiol and 2-hydroxyestradiol (2-OHE2). It is specifically the 2-methyl ether of 2-hydroxyestradiol. 2-Methoxyestradiol prevents the formation of new blood vessels that tumors need in order to grow (angiogenesis), hence it is an angiogenesis inhibitor. It also acts as a vasodilator and induces apoptosis in some cancer cell lines. 2-Methoxyestradiol is derived from estradiol, although it interacts poorly with the estrogen receptors. However, it retains activity as a high-affinity agonist of the G protein-coupled estrogen receptor (GPER).

Estrogen receptors (ERs) are proteins found in cells that function as receptors for the hormone estrogen (17β-estradiol). There are two main classes of ERs. The first includes the intracellular estrogen receptors, namely ERα and ERβ, which belong to the nuclear receptor family. The second class consists of membrane estrogen receptors (mERs), such as GPER (GPR30), ER-X, and Gq-mER, which are primarily G protein-coupled receptors. This article focuses on the nuclear estrogen receptors.

16α-Hydroxydehydroepiandrosterone is an endogenous metabolite of dehydroepiandrosterone (DHEA). Both 16α-OH-DHEA and its 3β-sulfate ester, 16α-OH-DHEA-S, are intermediates in the biosynthesis of estriol from dehydroepiandrosterone (DHEA). 16α-OH-DHEA has estrogenic activity.

Estetrol (E4), or oestetrol, is one of the four natural estrogenic steroid hormones found in humans, along with estrone (E1), estradiol (E2), and estriol (E3). Estetrol is a major estrogen in the body. In contrast to estrone and estradiol, estetrol is a native estrogen of fetal life. Estetrol is produced exclusively by the fetal liver and is found in detectable levels only during pregnancy, with relatively high levels in the fetus and lower levels in the maternal circulation.
17α-Estradiol is a minor and weak endogenous steroidal estrogen that is related to 17β-estradiol. It is the C17 epimer of estradiol. It has approximately 100-fold lower estrogenic potency than 17β-estradiol. The compound shows preferential affinity for the ERα over the ERβ. Although 17α-estradiol is far weaker than 17β-estradiol as an agonist of the nuclear estrogen receptors, it has been found to bind to and activate the brain-expressed ER-X with a greater potency than that of 17β-estradiol, suggesting that it may be the predominant endogenous ligand for the receptor.

20α-Dihydroprogesterone (20α-DHP), also known as 20α-hydroxyprogesterone (20α-OHP), is a naturally occurring, endogenous progestogen. It is a metabolite of progesterone, formed by the 20α-hydroxysteroid dehydrogenases (20α-HSDs) AKR1C1, AKR1C2, and AKR1C3 and the 17β-hydroxysteroid dehydrogenase (17β-HSD) HSD17B1. 20α-DHP can be transformed back into progesterone by 20α-HSDs and by the 17β-HSD HSD17B2. HSD17B2 is expressed in the human endometrium and cervix among other tissues. In animal studies, 20α-DHP has been found to be selectively taken up into and retained in target tissues such as the uterus, brain, and skeletal muscle.

16α-Iodo-E2, or 16α-iodoestradiol, is a synthetic, steroidal, potent estrogen with slight preference for the ERα over the ERβ that is used in scientific research. The KD of 16α-iodo-E2 for the ERα is 0.6 nM and for the ERβ is 0.24 nM, a 4-fold difference in affinity, whereas estradiol is considered to have similar affinity for the two receptor subtypes. Unlike the case of the much weaker estriol (16α-hydroxyestradiol), 16α-iodo-E2 is considered to be equipotent with estradiol in terms of estrogenic activity. Radiolabeled [16α-125I]iodo-E2 has been employed in imaging to study the estrogen receptor.

2-Hydroxyestradiol (2-OHE2), also known as estra-1,3,5(10)-triene-2,3,17β-triol, is an endogenous steroid, catechol estrogen, and metabolite of estradiol, as well as a positional isomer of estriol.

16β,17α-Epiestriol, or 16,17-epiestriol, also known as 16β-hydroxy-17α-estradiol, as well as estra-1,3,5(10)-triene-3,16β,17α-triol, is a minor and weak endogenous steroidal estrogen that is related to 17α-estradiol and estriol. Along with estriol, 16β,17α-epiestriol has been detected in the urine of women during the late pregnancy stage. It shows preferential affinity for the ERβ over the ERα.

16α-Hydroxyestrone (16α-OH-E1), or hydroxyestrone, also known as estra-1,3,5(10)-triene-3,16α-diol-17-one, is an endogenous steroidal estrogen and a major metabolite of estrone, as well as an intermediate in the biosynthesis of estriol. It is a potent estrogen similarly to estrone, and it has been suggested that the ratio of 16α-hydroxyestrone to 2-hydroxyestrone, the latter being much less estrogenic in comparison and even antiestrogenic in the presence of more potent estrogens like estradiol, may be involved in the pathophysiology of breast cancer. Conversely, 16α-hydroxyestrone may help to protect against osteoporosis.

A catechol estrogen is a steroidal estrogen that contains catechol (1,2-dihydroxybenzene) within its structure. The catechol estrogens are endogenous metabolites of estradiol and estrone and include the following compounds:

2-Hydroxyestrone (2-OHE1), also known as estra-1,3,5(10)-trien-2,3-diol-17-one, is an endogenous, naturally occurring catechol estrogen and a major metabolite of estrone and estradiol. It is formed irreversibly from estrone in the liver and to a lesser extent in other tissues via 2-hydroxylation mediated by cytochrome P450 enzymes, mainly the CYP3A and CYP1A subfamilies. 2-OHE1 is the most abundant catechol estrogen in the body.
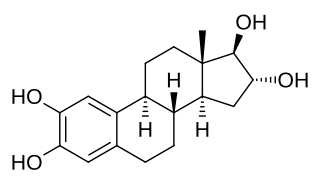
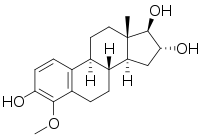
2-Hydroxyestriol, also known as estra-1,3,5(10)-triene-2,3,16α,17β-tetrol, is an endogenous catechol estrogen and metabolite of estriol. It is a suspected carcinogen of carcinogenicity category 2.

2-Methoxyestrone (2-ME1) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estrone that is formed by catechol O-methyltransferase via the intermediate 2-hydroxyestrone. Unlike estrone but similarly to 2-hydroxyestrone and 2-methoxyestradiol, 2-methoxyestrone has very low affinity for the estrogen receptor and lacks significant estrogenic activity.
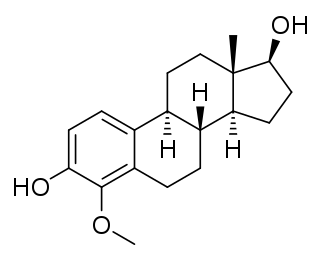
4-Methoxyestradiol (4-ME2) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estradiol that is formed by catechol O-methyltransferase via the intermediate 4-hydroxyestradiol. It has estrogenic activity similarly to estrone and 4-hydroxyestrone.

4-Methoxyestrone (4-ME1) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estrone that is formed by catechol O-methyltransferase via the intermediate 4-hydroxyestrone. It has estrogenic activity similarly to estrone and 4-hydroxyestrone.

The hydroxylation of estradiol is one of the major routes of metabolism of the estrogen steroid hormone estradiol. It is hydroxylated into the catechol estrogens 2-hydroxyestradiol and 4-hydroxyestradiol and into estriol (16α-hydroxyestradiol), reactions which are catalyzed by cytochrome P450 enzymes predominantly in the liver, but also in various other tissues.

2-Methoxyestriol (2-MeO-E3) is an endogenous estrogen metabolite. It is specifically a metabolite of estriol and 2-hydroxyestriol. It has negligible affinity for the estrogen receptors and no estrogenic activity. However, 2-methoxyestriol does have some non-estrogen receptor-mediated cholesterol-lowering effects.

Estradiol 3-glucuronide 17β-sulfate (E2-3G-17S) is an endogenous estrogen conjugate and metabolite of estradiol. It is related to estradiol 3-sulfate and estradiol 17β-glucuronide. Estradiol 3-glucuronide 17β-sulfate has 0.0001% of the relative binding affinity of estradiol for the ERα, one of the two estrogen receptors (ERs). It shows less than one million-fold lower potency in activating the estrogen receptors relative to estradiol in vitro.