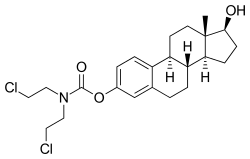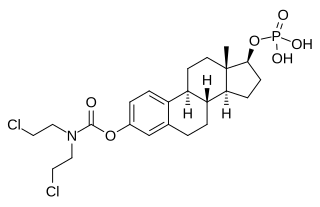
Estramustine phosphate (EMP), also known as estradiol normustine phosphate and sold under the brand names Emcyt and Estracyt, is a dual estrogen and chemotherapy medication which is used in the treatment of prostate cancer in men. It is taken multiple times a day by mouth or by injection into a vein.

Estriol succinate, sold under the brand name Synapause among others, is an estrogen medication which is used in the treatment of menopausal symptoms. It is taken by mouth, in through the vagina, and by injection.
Estradiol dipropionate (EDP), sold under the brand names Agofollin, Di-Ovocylin, and Progynon DP among others, is an estrogen medication which has been used in hormone therapy for menopausal symptoms and low estrogen levels in women and in the treatment of gynecological disorders. It has also been used in feminizing hormone therapy for transgender women and in the treatment of prostate cancer in men. Although widely used in the past, estradiol dipropionate has largely been discontinued and is mostly no longer available today. It appears to remain in use only in Japan, Macedonia, and Australia. Estradiol dipropionate is given by injection into muscle at intervals ranging from once or twice a week to once every week and a half to two weeks.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.

Orestrate, also known as estradiol 3-propionate 17β-(1-cyclohexenyl) ether, is an estrogen medication and estrogen ester which was never marketed. It is the C3 propionate ester and C17β-(1-cyclohexenyl) ether of estradiol.

Estradiol hexahydrobenzoate (EHHB), sold under a number of brand names including Benzo-Ginoestril A.P., BenzoGynoestryl Retard, Ginestryl-15-Depot, Menodin, and Tardoginestryl, is an estrogen medication which was previously used for indications such as menopausal hormone therapy and gynecological disorders. EHHB is given by injection into muscle at regular intervals, for instance once every few weeks.

Atrimustine, also known as bestrabucil or busramustine, is a cytostatic antineoplastic agent which was under development in Japan by Kureha Chemicals for the treatment of breast cancer and non-Hodgkin's lymphoma as well as for the prevention of graft-versus-host disease in bone marrow transplant recipients. It is the benzoate ester of an ester conjugate of estradiol and chlorambucil, which results in targeted/site-directed cytostatic activity toward estrogen receptor–positive tissues such as breast and bone. It reached preregistration for the treatment of cancer but was ultimately discontinued. Estrogenicic side effects of atrimustine in clinical trials included vaginal bleeding and gynecomastia. The drug was first patented in 1980.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

Alestramustine, also known as estradiol 3-(bis carbamate) 17β-(L-alaninate), is a cytostatic antineoplastic agent which was never marketed. It is the L-alanine ester of estramustine, which is a combination of the nitrogen mustard normustine coupled via a carbamate to the estrogen estradiol. Alestramustine acts as a prodrug to estramustine, and also forms estradiol as a byproduct. The drug, via its active metabolites, binds to microtubule-associated proteins and β-tubulin and interferes with microtubule function, thereby inhibiting cell division. Due to its estrogen moiety, alestramustine is selectively concentrated in estrogen receptor-positive cells such as prostate and breast.

ICI-85966, also known as diethylstilbestrol (DES) bis(di carbamate), is a synthetic, nonsteroidal estrogen and cytostatic antineoplastic agent of the stilbestrol group and a nitrogen mustard ester of diethylstilbestrol (DES) which was developed for the treatment of breast cancer and prostate cancer but was never marketed.

Estromustine, also known as estrone 17β-3-N-bis(2-chloroethyl)carbamate or estrone–cytostatic complex, is a major active metabolite of the cytostatic antineoplastic agent and estrogen estramustine phosphate, a medication used in the treatment of prostate cancer.
Phenestrol, or fenestrol, also known as hexestrol bis[4-[bis(2-chloroethyl)amino]phenylacetate, is a synthetic, nonsteroidal estrogen and cytostatic antineoplastic agent and a chlorphenacyl nitrogen mustard ester of hexestrol which was developed in the early 1960s for the treatment of hormone-dependent tumors but was never marketed.

Estradiol mustard, also known as estradiol 3,17β-bis(4- phenyl)acetate, is a semisynthetic, steroidal estrogen and cytostatic antineoplastic agent and a phenylacetic acid nitrogen mustard-coupled estrogen ester that was never marketed. It is selectively distributed into estrogen receptor (ER)-positive tissues such as ER-expressing tumors like those seen in breast and prostate cancers. For this reason, estradiol mustard and other cytostatic-linked estrogens like estramustine phosphate have reduced toxicity relative to non-linked nitrogen mustard cytostatic antineoplastic agents. However, they may stimulate breast tumor growth due to their inherent estrogenic activity and are said to be devoid of major therapeutic efficacy in breast cancer, although estramustine phosphate has been approved for and is used in the treatment of prostate cancer.
Cytestrol acetate is a steroidal antiestrogen and a cytostatic antineoplastic agent which was developed for the treatment of breast cancer but was never marketed.

Testifenon, also known as testiphenon, testiphenone, chlorphenacyl dihydrotestosterone ester, or dihydrotestosterone 17β-(4- phenyl)acetate, is a synthetic anabolic–androgenic steroid (AAS) and a cytostatic antineoplastic agent that was never marketed. It is an androgen ester – specifically, a chlorphenacyl nitrogen mustard ester of dihydrotestosterone (DHT) – and acts as a prodrug of these two components in the body. The drug was developed in Russia as a tissue-selective cytostatic drug for the treatment of various cancers occurring in androgen receptor-expressing tissues that would have reduced side effects and toxicity relative to other chemotherapy drugs.

Cortifen, also known as cortiphen or kortifen, as well as fencoron, is a synthetic glucocorticoid corticosteroid and cytostatic antineoplastic agent which was developed in Russia for potential treatment of tumors. It is a hydrophobic chlorphenacyl nitrogen mustard ester of 11-deoxycortisol (cortodoxone).
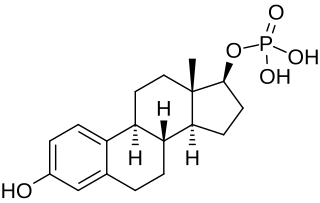
Estradiol phosphate, or estradiol 17β-phosphate, also known as estra-1,3,5(10)-triene-3,17β-diol 17β-(dihydrogen phosphate), is an estrogen which was never marketed. It is an estrogen ester, specifically an ester of estradiol with phosphoric acid, and acts as a prodrug of estradiol in the body. It is rapidly cleaved by phosphatase enzymes into estradiol upon administration. Estradiol phosphate is contained within the chemical structures of two other estradiol esters, polyestradiol phosphate and estramustine phosphate, both of which have been marketed for the treatment of prostate cancer.