
Chemotherapy is a type of cancer treatment that uses one or more anti-cancer drugs as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent or it may aim to prolong life or to reduce symptoms. Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called medical oncology.

Mitotane, sold under the brand name Lysodren, is a steroidogenesis inhibitor and cytostatic antineoplastic medication which is used in the treatment of adrenocortical carcinoma and Cushing's syndrome. It is a derivative of the early insecticide DDT and an isomer of p,p'-DDDTooltip dichlorodiphenyldichloroethane (4,4'-dichlorodiphenyldichloroethane) and is also known as 2,4'-(dichlorodiphenyl)-2,2-dichloroethane (o,p'-DDD).

Altretamine, also called hexamethylmelamine, is an antineoplastic agent. It was approved by the U.S. FDA in 1990.

Fluorometholone acetate, also known as oxylone acetate and sold under the brand names Flarex, Florate, and Omnitrol, is a synthetic glucocorticoid corticosteroid and a corticosteroid ester, as well as a progestogen and progestogen ester. It is the C17α acetate ester of fluorometholone.
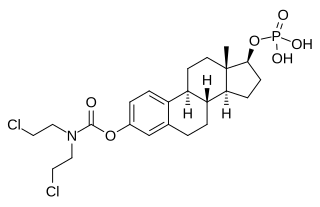
Estramustine phosphate (EMP), also known as estradiol normustine phosphate and sold under the brand names Emcyt and Estracyt, is a dual estrogen and chemotherapy medication which is used in the treatment of prostate cancer in men. It is taken multiple times a day by mouth or by injection into a vein.

Chlorotrianisene (CTA), also known as tri-p-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal symptoms and estrogen deficiency in women and prostate cancer in men, among other indications, but has since been discontinued and is now no longer available. It is taken by mouth.
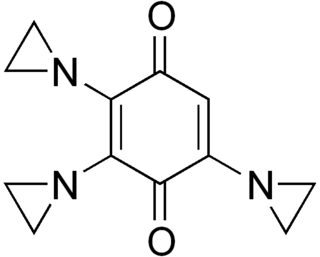
An alkylating antineoplastic agent is an alkylating agent used in cancer treatment that attaches an alkyl group (CnH2n+1) to DNA.
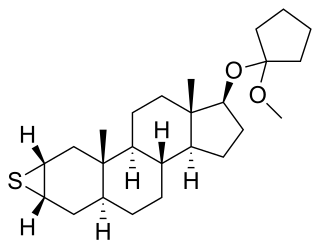
Mepitiostane, sold under the brand name Thioderon, is an orally active antiestrogen and anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which is marketed in Japan as an antineoplastic agent for the treatment of breast cancer. It is a prodrug of epitiostanol. The drug was patented and described in 1968.
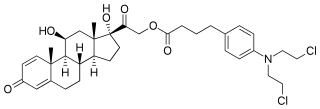
Prednimustine, sold under the brand names Mostarina and Sterecyst, is a medication which is used in chemotherapy in the treatment of leukemias and lymphomas. It is the ester formed from two other drugs, prednisolone and chlorambucil. Rarely, it has been associated with myoclonus.

Calusterone, also known as 7β,17α-dimethyltestosterone, is an orally active anabolic-androgenic steroid (AAS) that is used as an antineoplastic agent. It is a 17α-alkylated AAS similar in structure to bolasterone.

Epitiostanol, sold under the brand name Thiodrol, is an injected antiestrogen and anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was described in the literature in 1965 and has been marketed in Japan as an antineoplastic agent for the treatment of breast cancer since 1977.

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

Atrimustine, also known as bestrabucil or busramustine, is a cytostatic antineoplastic agent which was under development in Japan by Kureha Chemicals for the treatment of breast cancer and non-Hodgkin's lymphoma as well as for the prevention of graft-versus-host disease in bone marrow transplant recipients. It is the benzoate ester of an ester conjugate of estradiol and chlorambucil, which results in targeted/site-directed cytostatic activity toward estrogen receptor–positive tissues such as breast and bone. It reached preregistration for the treatment of cancer but was ultimately discontinued. Estrogenicic side effects of atrimustine in clinical trials included vaginal bleeding and gynecomastia. The drug was first patented in 1980.

Alestramustine, also known as estradiol 3-(bis carbamate) 17β-(L-alaninate), is a cytostatic antineoplastic agent which was never marketed. It is the L-alanine ester of estramustine, which is a combination of the nitrogen mustard normustine coupled via a carbamate to the estrogen estradiol. Alestramustine acts as a prodrug to estramustine, and also forms estradiol as a byproduct. The drug, via its active metabolites, binds to microtubule-associated proteins and β-tubulin and interferes with microtubule function, thereby inhibiting cell division. Due to its estrogen moiety, alestramustine is selectively concentrated in estrogen receptor-positive cells such as prostate and breast.

Estradiol mustard, also known as estradiol 3,17β-bis(4- phenyl)acetate, is a semisynthetic, steroidal estrogen and cytostatic antineoplastic agent and a phenylacetic acid nitrogen mustard-coupled estrogen ester that was never marketed. It is selectively distributed into estrogen receptor (ER)-positive tissues such as ER-expressing tumors like those seen in breast and prostate cancers. For this reason, estradiol mustard and other cytostatic-linked estrogens like estramustine phosphate have reduced toxicity relative to non-linked nitrogen mustard cytostatic antineoplastic agents. However, they may stimulate breast tumor growth due to their inherent estrogenic activity and are said to be devoid of major therapeutic efficacy in breast cancer, although estramustine phosphate has been approved for and is used in the treatment of prostate cancer.
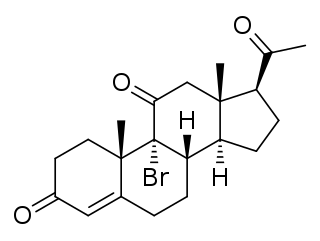
Bromoketoprogesterone (BKP), also known as 9α-bromo-11-oxoprogesterone (BOP), and known by the tentative brand name Braxarone (Squibb), is an orally active progestin which does not appear to have been marketed.

Panomifene is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group related to tamoxifen that was under development as an antineoplastic agent by Egis Pharmaceuticals and IVAX Drug Research Institute in the 1990s for the treatment of breast cancer, but it was never marketed. It reached phase II clinical trials before development was terminated. The drug was described in 1981.
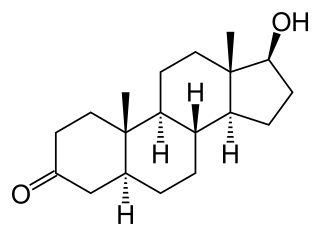
Androstanolone, or stanolone, also known as dihydrotestosterone (DHT) and sold under the brand name Andractim among others, is an androgen and anabolic steroid (AAS) medication and hormone which is used mainly in the treatment of low testosterone levels in men. It is also used to treat breast development and small penis in males. Compared to testosterone, androstanolone (DHT) is less likely to aromatize into estrogen, and therefore it shows less pronounced estrogenic side effects, such as gynecomastia and water retention. On the other hand, androstanolone (DHT) show more significant androgenic side effects, such as acne, hair loss and prostate enlargement.

5α-Dihydroethisterone is an active metabolite of the formerly clinically used but now-discontinued progestin ethisterone and the experimental and never-marketed hormonal antineoplastic agent ethynylandrostanediol (HE-3235). Its formation from its parent drugs is catalyzed by 5α-reductase in tissues that express the enzyme in high amounts like the liver, skin, hair follicles, and prostate gland. 5α-DHET has significant affinity for steroid hormone receptors and may contribute importantly to the activities of its parent drugs.



















