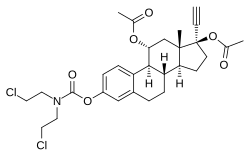
Estramustine phosphate (EMP), also known as estradiol normustine phosphate and sold under the brand names Emcyt and Estracyt, is a dual estrogen and chemotherapy medication which is used in the treatment of prostate cancer in men. It is taken multiple times a day by mouth or by injection into a vein.
An alkylating antineoplastic agent is an alkylating agent used in cancer treatment that attaches an alkyl group (CnH2n+1) to DNA.
Hormonal therapy in oncology is hormone therapy for cancer and is one of the major modalities of medical oncology, others being cytotoxic chemotherapy and targeted therapy (biotherapeutics). It involves the manipulation of the endocrine system through exogenous or external administration of specific hormones, particularly steroid hormones, or drugs which inhibit the production or activity of such hormones. Because steroid hormones are powerful drivers of gene expression in certain cancer cells, changing the levels or activity of certain hormones can cause certain cancers to cease growing, or even undergo cell death. Surgical removal of endocrine organs, such as orchiectomy and oophorectomy can also be employed as a form of hormonal therapy.
Mepitiostane, sold under the brand name Thioderon, is an orally active antiestrogen and anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which is marketed in Japan as an antineoplastic agent for the treatment of breast cancer. It is a prodrug of epitiostanol. The drug was patented and described in 1968.

Epitiostanol, sold under the brand name Thiodrol, is an injected antiestrogen and anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was described in the literature in 1965 and has been marketed in Japan as an antineoplastic agent for the treatment of breast cancer since 1977.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.
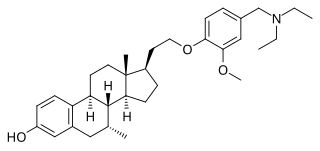
TAS-108, also known as SR-16234, is a drug discovered by Masato Tanabe and under development by SRI International and Taiho Pharmaceutical. It is a steroid hormone that has shown signs of treating and preventing breast cancer, even in patients where tamoxifen has failed.

Alestramustine, also known as estradiol 3-(bis carbamate) 17β-(L-alaninate), is a cytostatic antineoplastic agent which was never marketed. It is the L-alanine ester of estramustine, which is a combination of the nitrogen mustard normustine coupled via a carbamate to the estrogen estradiol. Alestramustine acts as a prodrug to estramustine, and also forms estradiol as a byproduct. The drug, via its active metabolites, binds to microtubule-associated proteins and β-tubulin and interferes with microtubule function, thereby inhibiting cell division. Due to its estrogen moiety, alestramustine is selectively concentrated in estrogen receptor-positive cells such as prostate and breast.

Acetomepregenol (ACM), also known as mepregenol diacetate and sold under the brand name Diamol, is a progestin medication which is used in Russia for the treatment of gynecological conditions and as a method of birth control in combination with an estrogen. It has also been studied in the treatment of threatened abortion. It has been used in veterinary medicine as well. It has been marketed since at least 1981.

ICI-85966, also known as diethylstilbestrol (DES) bis(di carbamate), is a synthetic, nonsteroidal estrogen and cytostatic antineoplastic agent of the stilbestrol group and a nitrogen mustard ester of diethylstilbestrol (DES) which was developed for the treatment of breast cancer and prostate cancer but was never marketed.
Phenestrol, or fenestrol, also known as hexestrol bis[4-[bis(2-chloroethyl)amino]phenylacetate, is a synthetic, nonsteroidal estrogen and cytostatic antineoplastic agent and a chlorphenacyl nitrogen mustard ester of hexestrol which was developed in the early 1960s for the treatment of hormone-dependent tumors but was never marketed.

Estradiol mustard, also known as estradiol 3,17β-bis(4- phenyl)acetate, is a semisynthetic, steroidal estrogen and cytostatic antineoplastic agent and a phenylacetic acid nitrogen mustard-coupled estrogen ester that was never marketed. It is selectively distributed into estrogen receptor (ER)-positive tissues such as ER-expressing tumors like those seen in breast and prostate cancers. For this reason, estradiol mustard and other cytostatic-linked estrogens like estramustine phosphate have reduced toxicity relative to non-linked nitrogen mustard cytostatic antineoplastic agents. However, they may stimulate breast tumor growth due to their inherent estrogenic activity and are said to be devoid of major therapeutic efficacy in breast cancer, although estramustine phosphate has been approved for and is used in the treatment of prostate cancer.

Butagest, also known as buterol, as well as 3β-hydroxy-6-methyl-17α-hydroxypregna-4,6-dien-20-one 3β-butanoate 17α-acetate or as 6-methyl-17α-hydroxy-δ6-progesterone 3β-butanoate 17α-acetate, is a steroidal progestin which was developed in Russia for potential clinical use but was never marketed. It is a modification of megestrol acetate in which the C3 ketone has been replaced with a C3β butanoyloxy moiety.

Testifenon, also known as testiphenon, testiphenone, chlorphenacyl dihydrotestosterone ester, or dihydrotestosterone 17β-(4- phenyl)acetate, is a synthetic anabolic–androgenic steroid (AAS) and a cytostatic antineoplastic agent that was never marketed. It is an androgen ester – specifically, a chlorphenacyl nitrogen mustard ester of dihydrotestosterone (DHT) – and acts as a prodrug of these two components in the body. The drug was developed in Russia as a tissue-selective cytostatic drug for the treatment of various cancers occurring in androgen receptor-expressing tissues that would have reduced side effects and toxicity relative to other chemotherapy drugs.

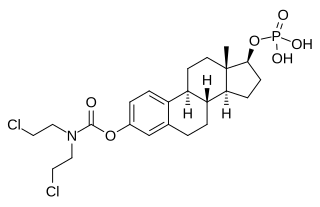
Estramustine is an estrogen and cytostatic antineoplastic agent which was never marketed. It is a carbamate derivative of estradiol and acts in part as a prodrug of estradiol in the body. Estramustine phosphate, the C17β phosphate ester of estramustine and a prodrug of estramustine, estromustine, estradiol, and estrone, is marketed and used in the treatment of prostate cancer.

Cortifen, also known as cortiphen or kortifen, as well as fencoron, is a synthetic glucocorticoid corticosteroid and cytostatic antineoplastic agent which was developed in Russia for potential treatment of tumors. It is a hydrophobic chlorphenacyl nitrogen mustard ester of 11-deoxycortisol (cortodoxone).

Cymegesolate, also known as cypionyl megestrol acetate or as megestrol acetate 3β-cypionate, is a progestin medication which was never marketed. It was developed in China in the late 1970s and early to mid 1980s for use as a hormonal contraceptive. The medication was formulated at a dose of 50–100 mg in combination with a "trace" dose of 0.25–0.5 mg quinestrol as a long-lasting, once-a-month combined oral contraceptive pill. This combination has been studied in 1,213 women across a total of 9,651 menstrual cycles, with contraceptive effectiveness of over 99.13% and "very few side effects." At the high dose, it showed an anovulation rate of only about 60%, and instead mediated its contraceptive effects via a marked anti-implantation effect.