
Clomifene, also known as clomiphene, is a medication used to treat infertility in women who do not ovulate, including those with polycystic ovary syndrome. It is taken by mouth.
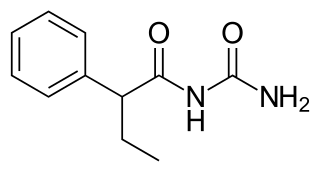
Pheneturide, also known as phenylethylacetylurea, is an anticonvulsant of the ureide class. Conceptually, it can be formed in the body as a metabolic degradation product from phenobarbital. It is considered to be obsolete and is now seldom used. It is marketed in Europe, including in Poland, Spain and the United Kingdom. Pheneturide has a similar profile of anticonvulsant activity and toxicity relative to phenacemide. As such, it is only used in cases of severe epilepsy when other, less-toxic drugs have failed. Pheneturide inhibits the metabolism and thus increases the levels of other anticonvulsants, such as phenytoin.

Dienestrol, also known as dienoestrol, is a synthetic nonsteroidal estrogen medication of the stilbestrol group which is or was used to treat menopausal symptoms in the United States and Europe. It has been studied for use by rectal administration in the treatment of prostate cancer in men as well. The medication was introduced in the U.S. in 1947 by Schering as Synestrol and in France in 1948 as Cycladiene. Dienestrol is a close analogue of diethylstilbestrol. It has approximately 223% and 404% of the affinity of estradiol at the ERα and ERβ, respectively.

Chlorotrianisene (CTA), also known as tri-p-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal symptoms and estrogen deficiency in women and prostate cancer in men, among other indications, but has since been discontinued and is now no longer available. It is taken by mouth.
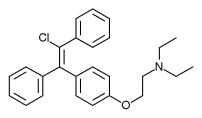
Enclomifene (INNTooltip International Nonproprietary Name), or enclomiphene (USANTooltip United States Adopted Name), a nonsteroidal selective estrogen receptor modulator of the triphenylethylene group, acts by antagonizing the estrogen receptor (ER) in the pituitary gland, which reduces negative feedback by estrogen on the hypothalamic-pituitary-gonadal axis, thereby increasing gonadotropin secretion and hence gonadal production of testosterone. It is one of the two stereoisomers of clomifene, which itself is a mixture of 38% zuclomifene and 62% enclomifene. Enclomifene is the (E)-stereoisomer of clomifene, while zuclomifene is the (Z)-stereoisomer. Whereas zuclomifene is more estrogenic, enclomifene is more antiestrogenic. In accordance, unlike enclomifene, zuclomifene is antigonadotropic due to activation of the ER and reduces testosterone levels in men. As such, isomerically pure enclomifene is more favorable than clomifene as a progonadotropin for the treatment of male hypogonadism.
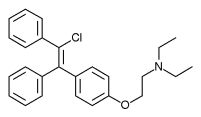
Zuclomifene (INN; or zuclomiphene (USAN)) is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was never marketed. It is one of the two stereoisomers of clomifene, which itself is a mixture of 38% zuclomifene and 62% enclomifene. Zuclomifene is the (Z)-stereoisomer of clomifene, while enclomifene is the (E)-stereoisomer. Whereas zuclomifene is described as mildly estrogenic, enclomifene is described as antiestrogenic. In accordance, unlike enclomifene, zuclomifene is antigonadotropic due to activation of the estrogen receptor and reduces testosterone levels in men. It is also about five times more potent than enclomifene in inducing ovulation.

Acecarbromal (INN), also known as acetylcarbromal and acetyladalin, is a hypnotic and sedative drug of the ureide (acylurea) group discovered by Bayer in 1917 that was formerly marketed in the United States and Europe. It is also used in combination with extract of quebracho and vitamin E as a treatment for erectile dysfunction under the brand name Afrodor in Europe. Acecarbromal is structurally related to the barbiturates, which are basically cyclized ureas. Prolonged use is not recommended as it can cause bromine poisoning.

Cyclofenil, sold under the brand name Sexovid among others, is a selective estrogen receptor modulator (SERM) medication which is used as a gonadotropin stimulant or ovulation inducer and in menopausal hormone therapy in women. It is mostly no longer available. The medication is taken by mouth.

Nafoxidine or nafoxidine hydrochloride is a nonsteroidal selective estrogen receptor modulator (SERM) or partial antiestrogen of the triphenylethylene group that was developed for the treatment of advanced breast cancer by Upjohn in the 1970s but was never marketed. It was developed at around the same time as tamoxifen and clomifene, which are also triphenylethylene derivatives. The drug was originally synthesized by the fertility control program at Upjohn as a postcoital contraceptive, but was subsequently repurposed for the treatment of breast cancer. Nafoxidine was assessed in clinical trials in the treatment of breast cancer and was found to be effective. However, it produced side effects including ichthyosis, partial hair loss, and phototoxicity of the skin in almost all patients, and this resulted in the discontinuation of its development.

Penmesterol, or penmestrol, also known as 17α-methyltestosterone 3-cyclopentyl enol ether, is a synthetic, orally active anabolic-androgenic steroid (AAS) that was developed in the early 1960s. It is the 3-cyclopentyl enol ether of methyltestosterone.
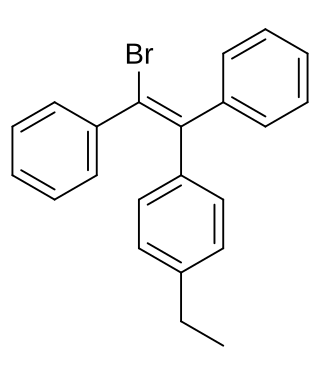
Broparestrol, also known as α-bromo-α,β-diphenyl-β-p-ethylphenylethylene (BDPE), is a synthetic, nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that has been used in Europe as a dermatological agent and for the treatment of breast cancer. The drug is described as slightly estrogenic and potently antiestrogenic, and inhibits mammary gland development and suppresses prolactin levels in animals. It is structurally related to clomifene and diethylstilbestrol. Broparestrol is a mixture of E- and Z- isomers, both of which are active, and are similarly antiestrogenic but, unlike broparestrol, were never marketed.

Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity. Its estrogenic effects were discovered in 1937. TPE was derived from structural modification of the more potent estrogen diethylstilbestrol, which is a member of the stilbestrol group of nonsteroidal estrogens.

Ethamoxytriphetol is a synthetic nonsteroidal antiestrogen that was studied clinically in the late 1950s and early 1960s but was never marketed. MER-25 was first reported in 1958, and was the first antiestrogen to be discovered. It has been described as "essentially devoid of estrogenic activity" and as having "very low estrogenic activity in all species tested". However, some estrogenic effects in the uterus have been observed, so it is not a pure antiestrogen but is, instead, technically a selective estrogen receptor modulator (SERM). For all intents and purposes, it is a nearly pure antiestrogen, however.

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.
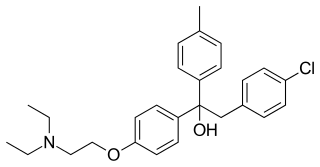
Triparanol was the first synthetic cholesterol-lowering drug. It was patented in 1959 and introduced in the United States in 1960. The developmental code name of triparanol, MER/29, became so well known that it became the registered trade name of the drug. It was withdrawn in 1962 due to severe adverse effects such as nausea and vomiting, vision loss due to irreversible cataracts, alopecia, skin disorders, and accelerated atherosclerosis. It is now considered to be obsolete.
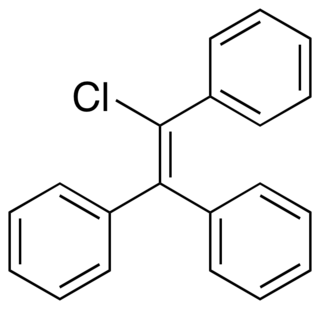
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.

Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed. A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.

Droloxifene, also known as 3-hydroxytamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed originally in Germany and later in Japan for the treatment of breast cancer, osteoporosis in men and postmenopausal women, and cardiovascular disorders but was abandoned and never marketed. It reached phase II and phase III clinical trials for these indications before development was discontinued in 2000. The drug was found to be significantly less effective than tamoxifen in the treatment of breast cancer in two phase III clinical trials.

Nitromifene (INNTooltip International Nonproprietary Name; also as the citrate salt nitromifene citrate (USANTooltip United States Adopted Name), developmental code names CI-628, CN-5518, CN-55945) is a nonsteroidal selective estrogen receptor modulator (SERM) related to triphenylethylenes like tamoxifen that was never marketed. It is a mixture of (E)- and (Z)-isomers that possess similar antiestrogenic activity. The drug was described in 1966. Along with tamoxifen, nafoxidine, and clomifene, it was one of the earliest SERMs.



















