Hot flashes are a form of flushing, often caused by the changing hormone levels that are characteristic of menopause. They are typically experienced as a feeling of intense heat with sweating and rapid heartbeat, and may typically last from two to 30 minutes for each occurrence.
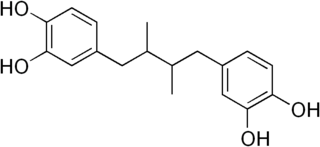
Nordihydroguaiaretic acid (NDGA) is a classic lignan, a phenylpropane dimer linked by a bond between positions C8 and C8′, as opposed to a neolignan. It is a natural compound found in the creosote bush.
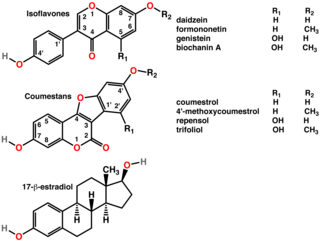
A phytoestrogen is a plant-derived xenoestrogen not generated within the endocrine system, but consumed by eating plants or manufactured foods. Also called a "dietary estrogen", it is a diverse group of naturally occurring nonsteroidal plant compounds that, because of its structural similarity to estradiol (17-β-estradiol), have the ability to cause estrogenic or antiestrogenic effects. Phytoestrogens are not essential nutrients because their absence from the diet does not cause a disease, nor are they known to participate in any normal biological function. Common foods containing phytoestrogens are soy protein, beans, oats, barley, rice, coffee, apples, carrots.

Equol (4',7-isoflavandiol) is an isoflavandiol estrogen metabolized from daidzein, a type of isoflavone found in soybeans and other plant sources, by bacterial flora in the intestines. While endogenous estrogenic hormones such as estradiol are steroids, equol is a nonsteroidal estrogen. Only about 30–50% of people have intestinal bacteria that make equol.
The lignans are a large group of low molecular weight polyphenols found in plants, particularly seeds, whole grains, and vegetables. The name derives from the Latin word for "wood". Lignans are precursors to phytoestrogens. They may play a role as antifeedants in the defense of seeds and plants against herbivores.
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones occur in many plant species, but are especially high in soybeans.

Secoisolariciresinol is an organic compound. It is classified as a lignan, i.e., a type of phenylpropanoid. It is present in some cereals, such as rye, and together with matairesinol has attracted much attention for its beneficial nutritional effects.

Genistein (C15H10O5) is a naturally occurring compound that structurally belongs to a class of compounds known as isoflavones. It is described as an angiogenesis inhibitor and a phytoestrogen.
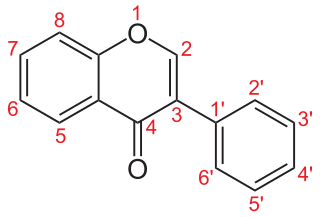
Isoflavonoids are a class of flavonoid phenolic compounds, many of which are biologically active. Isoflavonoids and their derivatives are sometimes referred to as phytoestrogens, as many isoflavonoid compounds have biological effects via the estrogen receptor.

Coumestrol is a natural organic compound in the class of phytochemicals known as coumestans. Coumestrol was first identified as a compound with estrogenic properties by E. M. Bickoff in ladino clover and alfalfa in 1957. It has garnered research interest because of its estrogenic activity and prevalence in some foods, including soybeans, brussels sprouts, spinach and a variety of legumes. The highest concentrations of coumestrol are found in clover, Kala Chana, and Alfalfa sprouts.

Sesamin is a lignan isolated from the bark of Fagara plants and from sesame oil. It has been used as a dietary fat-reduction supplement. Its major metabolite is enterolactone, which has an elimination half life of less than 6 hours. Sesamin and sesamolin are minor components of sesame oil, on average comprising only 0.14% of the oil by mass.

Enterolignans are organic compounds formed by the action of gut microflora on lignans. They are thus the products of the combined action of both plants and of the animal gut. Prominent enterolignans are enterodiol and enterolactone. Enterolignans are also called "mammalian lignans", although that term is self-contradictory since mammals do not produce lignans.

Enterolactone is a organic compound classified as an enterolignan. It is formed by the action of intestinal bacteria on plant lignan precursors present in the diet.

8-Prenylnaringenin (8-PN; also known as flavaprenin, (S)-8-dimethylallylnaringenin, hopein, or sophoraflavanone B) is a prenylflavonoid phytoestrogen. It is reported to be the most estrogenic phytoestrogen known. The compound is equipotent at the two forms of estrogen receptors, ERα and ERβ, and it acts as a full agonist of ERα. Its effects are similar to those of estradiol, but it is considerably less potent in comparison.
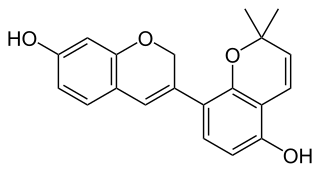
Glabrene is an isoflavonoid that is found in Glycyrrhiza glabra (licorice). It has estrogenic activity, showing estrogenic effects on breast, vascular, and bone tissue, and hence is a phytoestrogen (IC50 for estrogen receptor binding = 1 μM). It has also been found to act as a tyrosinase inhibitor (IC50 = 3.5 μM) and to inhibit the formation of melanin in melanocytes, and for these reasons, has been suggested as a potential skin-lightening agent.

Mirificin, also known as daidzein 8-C-(6-apiofuranosylglucoside), is an isoflavone that is found in Pueraria mirifica and Pueraria lobata. It has estrogenic activity and hence is a phytoestrogen.

Nyasol, also known as cis-hinokiresinol or as (Z)-hinokiresinol, is a lignan that is found in Anemarrhena asphodeloides. It has estrogenic activity, acting as a selective agonist of the ERβ, and hence is a phytoestrogen. In addition, (-)-nyasol has been found to inhibit the production of eicosanoids and nitric oxide in vitro and shows anti-inflammatory effects.
Sherwood Leslie Gorbach is an Emeritus Professor at Tufts University School of Medicine. He was editor-in-chief of the journal Clinical Infectious Diseases from 2000 to 2016.

Rimostil is a dietary supplement and extract of isoflavones from red clover which was under development by Kazia Therapeutics for the prevention of postmenopausal osteoporosis and cardiovascular disease and for the treatment of menopausal symptoms and hyperlipidemia but was never approved for medical use. It is enriched with isoflavone phytoestrogens such as formononetin, biochanin A, daidzein, and genistein, and is proposed to act as a selective estrogen receptor modulator, with both estrogenic and antiestrogenic effects in different tissues. The extract reached phase II clinical trials for cardiovascular disorders, hyperlipidemia, and postmenopausal osteoporosis prior to the discontinuation of its development in 2007.

Soy boy is a pejorative term sometimes used in online communities to describe men perceived to be lacking masculine characteristics. The term bears many similarities and has been compared to the slang terms cuck, nu-male and low-T – terms sometimes used as insults for male femininity by online communities.
















