
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

Gmelina arborea,, locally known as gamhar, is a fast-growing deciduous tree in the family Lamiaceae.

The Aza-Diels–Alder reaction is a modification of the Diels–Alder reaction wherein a nitrogen replaces sp2 carbon. The nitrogen atom can be part of the diene or the dienophile.

Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a natural product of the coumarin family.
The Bischler–Napieralski reaction is an intramolecular electrophilic aromatic substitution reaction that allows for the cyclization of β-arylethylamides or β-arylethylcarbamates. It was first discovered in 1893 by August Bischler and Bernard Napieralski, in affiliation with Basel Chemical Works and the University of Zurich. The reaction is most notably used in the synthesis of dihydroisoquinolines, which can be subsequently oxidized to isoquinolines.

Hypoglycin A is a naturally occurring amino acid derivative found in the unripened fruit of the Ackee tree and in the seeds of the box elder tree. It is toxic if ingested, and is the causative agent of Jamaican vomiting sickness. A 2017 Lancet report established a link between the consumption of unripened lychees resulting in hypoglycaemia and death from acute toxic encephalopathy.

In analytical chemistry, a chiral derivatizing agent (CDA), also known as a chiral resolving reagent, is a derivatization reagent that is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present and determine the optical purity of a sample. Analysis can be conducted by spectroscopy or by chromatography. Some analytical techniques such as HPLC and NMR, in their most commons forms, cannot distinguish enantiomers within a sample, but can distinguish diastereomers. Therefore, converting a mixture of enantiomers to a corresponding mixture of diastereomers can allow analysis. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.

Pinosylvin is an organic compound with the formula C6H5CH=CHC6H3(OH)2. A white solid, it is related to trans-stilbene, but with two hydroxy groups on one of the phenyl substituents. It is very soluble in many organic solvents, such as acetone.

Tropolone is an organic compound with the chemical formula C7H5(OH)O. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position.
Mosher's acid, or α-methoxy-α-trifluoromethylphenylacetic acid (MTPA) is a carboxylic acid which was first used by Harry Stone Mosher as a chiral derivatizing agent. It is a chiral molecule, consisting of R and S enantiomers.

Gmelina is a genus of plants in the family Lamiaceae. It consists of about 35 species in Australia, New Guinea, New Caledonia, Southeast Asia, India and a few in Africa. Some species such as G. arborea have been planted and/or become naturalised in India, Africa and Australia. It was named by Carl Linnaeus in honour of botanist Johann Georg Gmelin.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.

Arboreol is an epoxylignan. Arboreol can be transformed by acid catalysis into gmelanone.
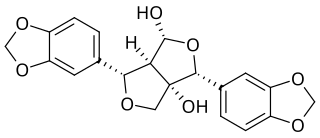
Gummadiol is a lignan hemiacetal. It can be isolated from the heartwood of Gmelina arborea.

Gmelinol is a lignan. (+)-Gmelinol can be isolated from the heartwood of Gmelina arborea. This compound, along with four other chemicals also found in the same species, (+)-7′-O-ethyl arboreol, (+)-paulownin, (+)-epieudesmin and (−)-β-sitosterol, shows antifungal activity against Trametes versicolor.
The Chan–Lam coupling reaction – also known as the Chan–Evans–Lam coupling is a cross-coupling reaction between an aryl boronic acid and an alcohol or an amine to form the corresponding secondary aryl amines or aryl ethers, respectively. The Chan–Lam coupling is catalyzed by copper complexes. It can be conducted in air at room temperature. The more popular Buchwald–Hartwig coupling relies on the use of palladium.

Caffeic aldehyde is a phenolic aldehyde contained in the seeds of Phytolacca americana. It is present in various parts of a large number of plants, such as the seeds of Phytolacca americana.
Ganugapati Sree Rama Subba Rao is an Indian natural product chemist and a former chair of the department of sciences at the Indian Institute of Science (IISc). He is known for his researches on dihydroaromatics obtained through Birch reduction of aromatic compounds and is an elected fellow of the Indian National Science Academy, and the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1982, for his contributions to chemical sciences.
Barry Ramachandra Rao was an Indian space physicist and the vice chairman of the University Grants Commission of India. Known for his pioneering research in radio physics, Rao was a Member of Parliament of the Rajya Sabha and an elected fellow of all the three major Indian science academies viz. Indian Academy of Sciences, Indian National Science Academy and National Academy of Sciences, India. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards for his contributions to Physical Sciences in 1965.

The epoxidation of allylic alcohols is a class of epoxidation reactions in organic chemistry. One implementation of this reaction is the Sharpless epoxidation. Early work showed that allylic alcohols give facial selectivity when using meta-chloroperoxybenzoic acid (m-CPBA) as an oxidant. This selectivity was reversed when the allylic alcohol was acetylated. This finding leads to the conclusion that hydrogen bonding played a key role in selectivity and the following model was proposed.















