 | |
| Names | |
|---|---|
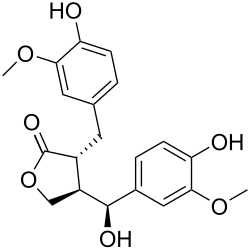
| IUPAC name (7′S,8β,8′α)-4,4′,7′-Trihydroxy-3,3′-dimethoxylignano-9,9′-lactone | |
| Systematic IUPAC name (3R,4R)-4-[(S)-Hydroxy(4-hydroxy-3-methoxyphenyl)methyl]-3-[(4-hydroxy-3-methoxyphenyl)methyl]oxolan-2-one | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C20H22O7 | |
| Molar mass | 374.389 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Hydroxymatairesinol (HMR) is a lignan found in Norway spruce (Picea abies). [1] It is an enterolactone precursor with anticancer activities. In rats, HMR decreased the volume of induced tumours and stabilised established tumours, as well as preventing the development of new tumours. [1] It has also shown anti-oxidant properties in vitro. [1]
HMR's chemical structure is similar to matairesinol. [2] At high concentrations, HMR has estrogenic properties, which are considerably weaker than those of estradiol. [2]