Electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons.
This is a list of the various reported boiling points for the elements, with recommended values to be used elsewhere on Wikipedia.
In chemical engineering, process design is the choice and sequencing of units for desired physical and/or chemical transformation of materials. Process design is central to chemical engineering, and it can be considered to be the summit of that field, bringing together all of the field's components.
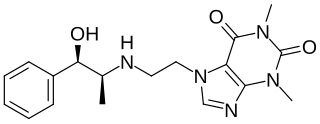
Cafedrine, sold under the brand name Akrinor among others, is a chemical linkage of norephedrine and theophylline and is a cardiac stimulant and antihypotensive agent used to increase blood pressure in people with hypotension. It has been marketed in Europe, South Africa, and Indonesia.
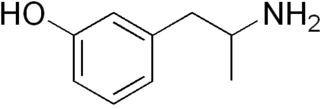
Gepefrine, also known as 3-hydroxyamphetamine or α-methyl-meta-tyramine and sold under the brand names Pressionorm and Wintonin, is a sympathomimetic medication used as an antihypotensive agent which has been marketed in Germany.
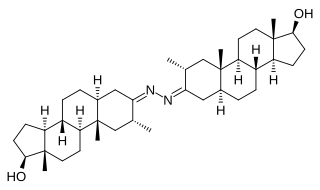
Bolazine, also known as 2α-methyl-5α-androstan-17β-ol-3-one azine, is a synthetic androgen/anabolic steroid (AAS) of the dihydrotestosterone (DHT) group which was never marketed. It is not orally active and is used as the ester prodrug bolazine capronate via depot intramuscular injection. Bolazine has a unique and unusual chemical structure, being a dimer of drostanolone linked at the C3 position of the A-ring by an azine group, and reportedly acts as a prodrug of drostanolone.
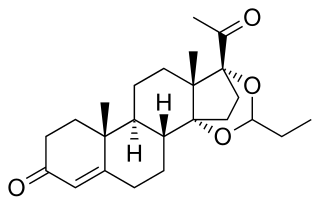
Proligestone, sold under the brand names Covinan and Delvosteron, is a progestin medication which is used in veterinary medicine.

Metaterol, also known as isofenefrine, isopropylnoradrianol, and 3,β-dihydroxy-N-isopropylphenethylamine, is a sympathomimetic and bronchodilator of the phenethylamine family that was never marketed. It is structurally related to norfenefrine, phenylephrine, and etilefrine.

Penmesterol, or penmestrol, also known as 17α-methyltestosterone 3-cyclopentyl enol ether, is a synthetic, orally active anabolic-androgenic steroid (AAS) that was developed in the early 1960s. It is the 3-cyclopentyl enol ether of methyltestosterone.
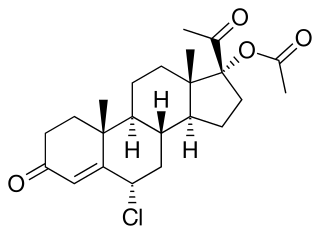
Hydromadinone acetate, also known as chloroacetoxyprogesterone (CAP), as well as 6α-chloro-17α-acetoxyprogesterone or 6α-chloro-17α-acetoxypregn-4-ene-3,20-dione, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. It is the C17α acetate ester of hydromadinone, which, similarly, was never marketed.
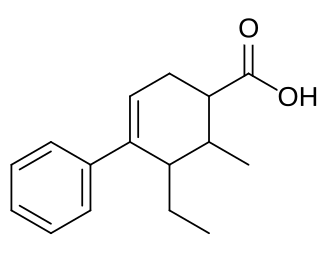
Fenestrel is a synthetic, nonsteroidal estrogen that was developed as a postcoital contraceptive in the 1960s but was never marketed. Synthesized by Ortho Pharmaceutical in 1961 and studied extensively, it was coined the "morning-after-pill" or "postcoital antifertility agent". Fenestrel is a seco analogue of doisynolic acid, and a member of the cyclohexenecarboxylic acid series of estrogens.
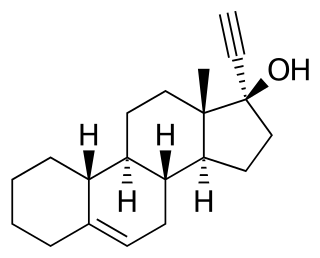
Cingestol, also known as 17α-ethynylestr-5-en-17β-ol, is a steroidal progestin of the 19-nortestosterone group that was never marketed. It was synthesized in 1969 and was developed in the 1970s by Organon as a low-dose, progestogen-only contraceptive, but in 1984, was still described as "under investigation". The drug is an isomer of lynestrenol with the double bond between C5 and C6.

Furostilbestrol (INN), also known as diethylstilbestrol di(2-furoate) or simply as diethylstilbestrol difuroate, is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol, that was never marketed. It is an ester of diethylstilbestrol and was described in the literature in 1952.
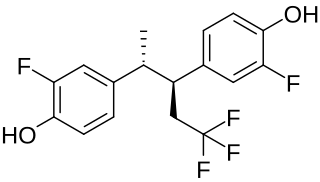
Pentafluranol is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol that was developed for the treatment of benign prostatic hyperplasia never marketed. It was described in the medical literature in 1974.

Taleranol, or teranol, also known as β-zearalanol, is a synthetic, nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in Fusarium spp which was never marketed. It is the β epimer of zeranol (α-zearalanol) and is a major metabolite of zeranol but with less biological activity.

Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed. A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.
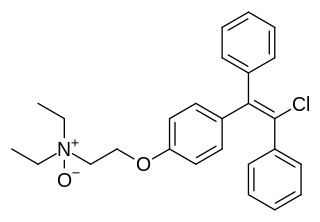
Clomifenoxide (INN), also known as clomifene N-oxide, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that is described as an antiestrogen and "gonad stimulant" and was never marketed. It is an active metabolite of clomifene.

Androstenediol dipropionate, or 5-androstenediol 3β,17β-dipropionate, also known as androst-5-ene-3β,17β-diol 3β,17β-dipropionate, is a synthetic anabolic–androgenic steroid and an androgen ester – specifically, the dipropionate diester of 5-androstenediol (androst-5-ene-3β,17β-diol) – which has been marketed in Europe, including in Spain, Italy, and Austria.

Timobesone is a synthetic glucocorticoid corticosteroid which was never marketed.
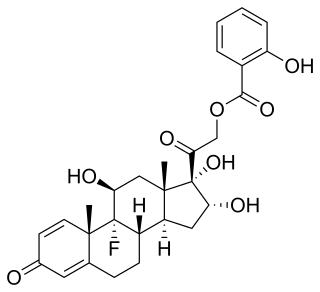
Cortobenzolone, also known as betamethasone salicylate, is a synthetic glucocorticoid corticosteroid and corticosteroid ester which is marketed in Spain.

















