
Clomifene, also known as clomiphene, is a medication used to treat infertility in women who do not ovulate, including those with polycystic ovary syndrome. It is taken by mouth.

Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonists/antagonists (ERAAs), are a class of drugs that act on estrogen receptors (ERs). Compared to pure ER agonists–antagonists, SERMs are more tissue-specific, allowing them to selectively inhibit or stimulate estrogen-like action in various tissues.
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative. Nonsteroidal anti-inflammatory drugs (NSAIDs) are distinguished from corticosteroids as a class of anti-inflammatory agents.

Chlorotrianisene (CTA), also known as tri-p-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal symptoms and estrogen deficiency in women and prostate cancer in men, among other indications, but has since been discontinued and is now no longer available. It is taken by mouth.

Zuclomifene (INN; or zuclomiphene (USAN)) is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was never marketed. It is one of the two stereoisomers of clomifene, which itself is a mixture of 38% zuclomifene and 62% enclomifene. Zuclomifene is the (Z)-stereoisomer of clomifene, while enclomifene is the (E)-stereoisomer. Whereas zuclomifene is described as mildly estrogenic, enclomifene is described as antiestrogenic. In accordance, unlike enclomifene, zuclomifene is antigonadotropic due to activation of the estrogen receptor and reduces testosterone levels in men. It is also about five times more potent than enclomifene in inducing ovulation.

Afimoxifene, also known as 4-hydroxytamoxifen (4-OHT) and by its tentative brand name TamoGel, is a selective estrogen receptor modulator (SERM) of the triphenylethylene group and an active metabolite of tamoxifen. The drug is under development under the tentative brand name TamoGel as a topical gel for the treatment of hyperplasia of the breast. It has completed a phase II clinical trial for cyclical mastalgia, but further studies are required before afimoxifene can be approved for this indication and marketed.

Cyclofenil, sold under the brand name Sexovid among others, is a selective estrogen receptor modulator (SERM) medication which is used as a gonadotropin stimulant or ovulation inducer and in menopausal hormone therapy in women. It is mostly no longer available. The medication is taken by mouth.

Trimegestone, sold under the brand names Ondeva and Totelle among others, is a progestin medication which is used in menopausal hormone therapy and in the prevention of postmenopausal osteoporosis. It was also under development for use in birth control pills to prevent pregnancy, but ultimately was not marketed for this purpose. The medication is available alone or in combination with an estrogen. It is taken by mouth.

3β-Androstanediol, also known as 5α-androstane-3β,17β-diol, and sometimes shortened in the literature to 3β-diol, is an endogenous steroid hormone and a metabolite of androgens like dehydroepiandrosterone (DHEA) and dihydrotestosterone (DHT).

A nonsteroidal estrogen is an estrogen with a nonsteroidal chemical structure. The most well-known example is the stilbestrol estrogen diethylstilbestrol (DES). Although nonsteroidal estrogens formerly had an important place in medicine, they have gradually fallen out of favor following the discovery of toxicities associated with high-dose DES starting in the early 1970s, and are now almost never used. On the other hand, virtually all selective estrogen receptor modulators (SERMs) are nonsteroidal, with triphenylethylenes like tamoxifen and clomifene having been derived from DES, and these drugs remain widely used in medicine for the treatment of breast cancer among other indications. In addition to pharmaceutical drugs, many xenoestrogens, including phytoestrogens, mycoestrogens, and synthetic endocrine disruptors like bisphenol A, are nonsteroidal substances with estrogenic activity.

Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity. Its estrogenic effects were discovered in 1937. TPE was derived from structural modification of the more potent estrogen diethylstilbestrol, which is a member of the stilbestrol group of nonsteroidal estrogens.

Ethamoxytriphetol is a synthetic nonsteroidal antiestrogen that was studied clinically in the late 1950s and early 1960s but was never marketed. MER-25 was first reported in 1958, and was the first antiestrogen to be discovered. It has been described as "essentially devoid of estrogenic activity" and as having "very low estrogenic activity in all species tested". However, some estrogenic effects in the uterus have been observed, so it is not a pure antiestrogen but is, instead, technically a selective estrogen receptor modulator (SERM). For all intents and purposes, it is a nearly pure antiestrogen, however.
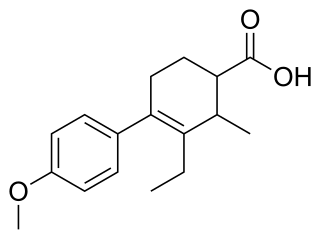
Carbestrol is a synthetic, nonsteroidal estrogen of the cyclohexenecarboxylic acid group and seco analogue of doisynolic acid that was described in the literature in 1956 and developed for the treatment of prostate cancer in the 1960s but was never marketed.

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

Doisynoestrol, also known as fenocycline, as well as cis-bisdehydrodoisynolic acid 7-methyl ether, is a synthetic nonsteroidal estrogen of the doisynolic acid group that is no longer marketed. It is a methyl ether of bisdehydrodoisynolic acid. Doisynoestrol was described in the literature in 1945. It has about 0.02% of the relative binding affinity of estradiol for the estrogen receptor.

Allenolic acid, or allenoic acid, is a synthetic, nonsteroidal estrogen discovered in 1947 or 1948 that, although studied clinically, was never marketed. It is an open-ring or seco-analogue of steroidal estrogens like estrone and equilenin. The compound was named after Edgar Allen, one of the pioneers in estrogen research. Although described as an estrogen, allenolic acid probably is totally inactive at the receptor, whereas a derivative, allenestrol, is reported to be a potent estrogen. Another derivative of allenolic acid, methallenestril, is also a potent estrogen and, in contrast to allenolic acid and allenestrol, has been marketed.

Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed. A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.

Droloxifene, also known as 3-hydroxytamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed originally in Germany and later in Japan for the treatment of breast cancer, osteoporosis in men and postmenopausal women, and cardiovascular disorders but was abandoned and never marketed. It reached phase II and phase III clinical trials for these indications before development was discontinued in 2000. The drug was found to be significantly less effective than tamoxifen in the treatment of breast cancer in two phase III clinical trials.

The pharmacology of bicalutamide is the study of the pharmacodynamic and pharmacokinetic properties of the nonsteroidal antiandrogen (NSAA) bicalutamide. In terms of pharmacodynamics, bicalutamide acts as a selective antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). It has no capacity to activate the AR. It does not decrease androgen levels and has no other important hormonal activity. The medication has progonadotropic effects due to its AR antagonist activity and can increase androgen, estrogen, and neurosteroid production and levels. This results in a variety of differences of bicalutamide monotherapy compared to surgical and medical castration, such as indirect estrogenic effects and associated benefits like preservation of sexual function and drawbacks like gynecomastia. Bicalutamide can paradoxically stimulate late-stage prostate cancer due to accumulated mutations in the cancer. When used as a monotherapy, bicalutamide can induce breast development in males due to its estrogenic effects. Unlike other kinds of antiandrogens, it may have less adverse effect on the testes and fertility.
A sex-hormonal agent, also known as a sex-hormone receptor modulator, is a type of hormonal agent which specifically modulates the effects of sex hormones and of their biological targets, the sex hormone receptors. The sex hormones include androgens such as testosterone, estrogens such as estradiol, and progestogens such as progesterone. Sex-hormonal agents may be either steroidal or nonsteroidal in chemical structure and may serve to either enhance, inhibit, or have mixed effects on the function of the sex hormone systems.


















