
Diethylstilbestrol (DES), also known as stilbestrol or stilboestrol, is a nonsteroidal estrogen medication, which is presently rarely used. In the past, it was widely used for a variety of indications, including pregnancy support for those with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency, treatment of prostate cancer and breast cancer, and other uses. By 2007, it was only used in the treatment of prostate cancer and breast cancer. In 2011, Hoover and colleagues reported on adverse health outcomes linked to DES including infertility, miscarriage, ectopic pregnancy, preeclampsia, preterm birth, stillbirth, infant death, menopause prior to age 45, breast cancer, cervical cancer, and vaginal cancer. While most commonly taken by mouth, DES was available for use by other routes as well, for instance, vaginal, topical, and by injection.

Fosfestrol, sold under the brand name Honvan and also known as diethylstilbestrol diphosphate (DESDP), is an estrogen medication which is used in the treatment of prostate cancer in men. It is given by slow intravenous infusion once per day to once per week or by mouth once per day.

Estriol succinate, sold under the brand name Synapause among others, is an estrogen medication which is used in the treatment of menopausal symptoms. It is taken by mouth, in through the vagina, and by injection.
An antigonadotropin is a drug which suppresses the activity and/or downstream effects of one or both of the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This results in an inhibition of the hypothalamic-pituitary-gonadal (HPG) axis, and thus a decrease in the levels of the androgen, estrogen, and progestogen sex steroids in the body. Antigonadotropins also inhibit ovulation in women and spermatogenesis in men. They are used for a variety of purposes, including for the hormonal birth control, treatment of hormonally-sensitive cancers, to delay precocious puberty and puberty in transgender youth, as a form of chemical castration to reduce the sex drives of individuals with hypersexuality or pedophilia, and to treat estrogen-associated conditions in women such as menorrhagia and endometriosis, among others. High-dose antigonadotropin therapy has been referred to as medical castration.

Hexestrol, sold under the brand name Synestrol among others, is a nonsteroidal estrogen which was previously used for estrogen replacement therapy and in the treatment of certain hormone-dependent cancers as well as gynecological disorders but is mostly no longer marketed. It has also been used in the form of esters such as hexestrol diacetate and hexestrol dipropionate. Hexestrol and its esters are taken by mouth, held under the tongue, or via injection into muscle.

Dienestrol diacetate is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It is an ester of dienestrol.

Diethylstilbestrol dipropionate (DESDP), or diethylstilbestrol dipropanoate, also known as stilboestrol dipropionate, is a synthetic nonsteroidal estrogen of the stilbestrol group that was formerly marketed widely throughout Europe. It is an ester of diethylstilbestrol with propionic acid, and is more slowly absorbed in the body than diethylstilbestrol. The medication has been said to be one of the most potent estrogens known.
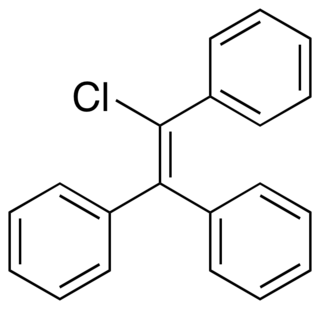
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.
ERA-45 is a synthetic estrogen and a selective agonist of the ERα. It shows 286-fold selectivity for transactivation of the ERα over the ERβ, with EC50 values of 0.37 nM for the ERα (7-fold weaker than estradiol) and 13 nM for the ERβ (20,000-fold weaker than estradiol). However, another found only about 35-fold potency for transactivation of the ERα over the ERβ. The drug has no antagonistic activity at either receptor. ERA-45 induced prostate cancer development in preclinical models when it was given in combination with testosterone, whereas testosterone alone did not do so. In contrast, the selective ERβ agonist ERB-26 was protective against the development of prostate cancer produced by these two drugs. These findings suggest opposing roles of the ERα and ERβ in the prostate gland. The chemical structure of ERa-45 does not appear to have been disclosed.

An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.

Estetrol (E4) is an estrogen medication and naturally occurring steroid hormone which is used in combination with a progestin in combined birth control pills and is under development for various other indications. These investigational uses include menopausal hormone therapy to treat symptoms such as vaginal atrophy, hot flashes, and bone loss and the treatment of breast cancer and prostate cancer. It is taken by mouth.

DU-41164, also known as 1,2β-methylene-6-fluoro-17α-acetoxy-δ6-retroprogesterone, is a progestin which was developed by Philips-Duphar in the 1970s and was never marketed. It is a combined derivative of 17α-hydroxyprogesterone and retroprogesterone. The drug shows extremely high potency as a progestogen in animals; it was reported to possess 500 times the affinity of progesterone for the progesterone receptor expressed in rabbit uterus, and showed 600 times the progestogenic potency of subcutaneous progesterone when given orally in animals. The affinity of DU-41164 for the progesterone receptor was described in 1974 as "probably the highest reported for any steroid-receptor interaction". The drug showed no androgenic, anabolic, antiandrogenic, estrogenic, or corticosteroid activity in animals. Although highly potent in animals, DU-41164 produced little or no progestogenic effect at dosages of 50 and 200 μg/day in women, suggesting major species differences. A closely related compound, DU-41165, has been developed as a photoaffinity label for the progesterone receptor.
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

Dimethylstilbestrol (DMS) is a nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol which was never marketed. It is a so-called "weak", "impeded", or "short-acting" estrogen similarly to estriol and meso-butoestrol. The affinity of DMS for the ER was reported as about 10% of that of estradiol. For comparison, diethylstilbestrol had 140% of the affinity of estradiol for the ER.

Polyestriol phosphate, sold under the brand names Gynäsan, Klimadurin, and Triodurin, is an estrogen medication which was previously used in menopausal hormone therapy and is no longer available.

Polydiethylstilbestrol phosphate is an estrogen medication which has been used in scientific research and has been studied for use in veterinary medicine as a livestock growth promoter. It is a phosphate ester of diethylstilbestrol (DES) in the form of a polymer and is a polymeric form of fosfestrol ; PDSP acts as a long-lasting prodrug of DES. It has similarities to polyestradiol phosphate and polyestriol phosphate.

The pharmacology of cyproterone acetate (CPA) concerns the pharmacology of the steroidal antiandrogen and progestin medication cyproterone acetate.
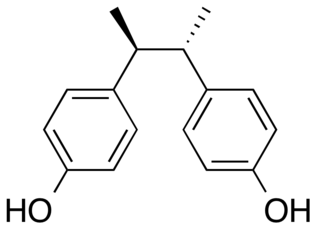
Butestrol, or racemic butestrol (rac-butestrol) is a synthetic nonsteroidal estrogen which was never marketed. It is structurally related to diethylstilbestrol and other stilbestrols.

ent-Estradiol (ent-E2), or 1-estradiol (1-E2), is an estrogen and the 1-enantiomorph of estradiol. It is a so-called "short-acting" or "impeded" estrogen, similarly to estriol, 17α-estradiol, and dimethylstilbestrol.
















