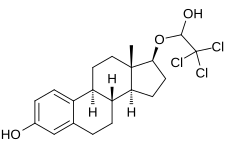
Cloxestradiol acetate, also known as 17-(2,2,2-trichloroethoxy)estradiol O,O-diacetate, is a synthetic steroidal estrogen derived from estradiol. It is the O,O-diacetate ester of cloxestradiol, which, in contrast to cloxestradiol acetate, was never marketed.

Estrapronicate, also known as estradiol nicotinate propionate is an estrogen medication and estrogen ester which was never marketed. It was studied as a component of the experimental tristeroid combination drug Trophobolene, which contained nandrolone decanoate, estrapronicate, and hydroxyprogesterone heptanoate.

Orestrate, also known as estradiol 3-propionate 17β-(1-cyclohexenyl) ether, is an estrogen medication and estrogen ester which was never marketed. It is the C3 propionate ester and C17β-(1-cyclohexenyl) ether of estradiol.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.

Estradiol butyrylacetate (EBA), sold under the brand names Follikosid and Klimanosid-R Depot, is an estrogen medication which is no longer marketed. It is an estrogen ester, specifically, an ester of estradiol. It is administered by intramuscular injection and a single 10 mg dose has been said to have a duration of action of 2 to 3 weeks. The excretion of EBA in women has been studied.
Estradiol diundecylate, or estradiol diundecanoate, also known as 17β-estradiol 3,17β-diundecylate, is an estrogen and an estrogen ester – specifically, the 3,17β-diundecylate ester of estradiol – which has been marketed in Romania. It was described, along with a variety of other estradiol esters such as estradiol undecylate, by Karl Junkmann of Schering AG in 1953.

Estradiol furoate (EF), or estradiol 17β-furoate, sold under the brand name Di-Folliculine, is an estrogen medication and estrogen ester which is no longer marketed. It is the C17β furoate ester of estradiol. Estradiol benzoate has also been marketed under the brand name Di-Folliculine, and should not be confused with estradiol furoate.

Estradiol propionate (EP), also known as estradiol monopropionate or estradiol 17β-propionate and sold under the brand names Acrofollin, Akrofollin, and Follhormon, is an estrogen medication and estrogen ester which is no longer marketed. It is the C17β propionate ester of estradiol. EP was provided in an oil solution and was administered by intramuscular injection. The medication was first marketed by 1938 or 1939.
Estradiol palmitate, or estradiol monopalmitate, also known as estradiol 17β-hexadecanoate, is a naturally occurring steroidal estrogen and an estrogen ester – specifically, the C17β palmitate ester of estradiol. It occurs in the body as a very long-lasting metabolite and prohormone of estradiol. The compound has no affinity for the estrogen receptor, requiring transformation into estradiol for its estrogenic activity. In addition to its endogenous role, estradiol palmitate was formerly used as a fattening agent in chickens under the brand name Esmopal.

Estradiol propoxyphenylpropionate (EPPP), sold under the brand name Durovex, is an estrogen medication and estrogen ester which is no longer marketed. It is the C17β propoxyphenylpropionate (propoxyhydrocinnamate) ester of estradiol. It is a long-acting depot estrogen.
Estradiol stearate (E2-17-St), also known as estradiol octadecanoate and sold under the brand name Depofollan, is a naturally occurring estrogen and an estrogen ester – specifically, the C17β stearate ester of estradiol. It occurs in the body as a very long-lasting metabolite and prohormone of estradiol. The compound is one of the components that collectively constitute lipoidal estradiol, another of which is estradiol palmitate. It is extremely lipophilic and hydrophobic. Estradiol stearate has no affinity for the estrogen receptor, requiring transformation into estradiol via esterases for its estrogenic activity. The compound does not bind to sex hormone-binding globulin or α-fetoprotein, instead being transported by lipoproteins such as high-density lipoprotein and low-density lipoprotein.

Estradiol hemisuccinate, or simply estradiol succinate, also known as estradiol 17β-hemisuccinate, is an estrogen medication and an estrogen ester – specifically, the hemisuccinate ester of estradiol. It is used as a component of hormone replacement therapy for menopause. Like other estrogens, estradiol hemisuccinate has been found to have beneficial effects on the skin, with improvement of skin thickness observed.

Estradiol pivalate, also known as estradiol trimethyl acetate (E2-TMA) and sold under the brand name Estrotate, is an estrogen medication and an estrogen ester; specifically, a pivalic acid ester of estradiol. Literature sources are conflicting as to whether the ester is located at the C3 position or at the C17β position. It was marketed as an oil solution for intramuscular injection in the 1940s and 1950s. A combination of estradiol pivalate (1 mg/mL) and progesterone (10 mg/mL) in oil solution for intramuscular injection was available in 1949. An aqueous suspension of estradiol pivalate was also developed by 1950 although whether it was ever marketed is unclear.

Estradiol 3-propionate, or 3-propanoylestradiol, also known as estra-1,3,5(10)-triene-3,17β-diol 3-propionate, is a semisynthetic, steroidal estrogen that was never marketed. It is an estrogen ester, specifically, a propionic acid ester of estradiol, and acts as a prodrug to it in vivo. The chemical structure of estradiol 3-propionate is contained within estradiol dipropionate, estrapronicate, and orestrate, all of which are also estradiol esters.
Estradiol diundecylenate, or estradiol diundecenoate, also known as 17β-estradiol 3,17β-diundec-10-enoate, is a semisynthetic steroidal estrogen and an estrogen ester – specifically, the 3,17β-diundecylenate ester of estradiol – which was previously marketed in Argentina.

Estradiol mustard, also known as estradiol 3,17β-bis(4- phenyl)acetate, is a semisynthetic, steroidal estrogen and cytostatic antineoplastic agent and a phenylacetic acid nitrogen mustard-coupled estrogen ester that was never marketed. It is selectively distributed into estrogen receptor (ER)-positive tissues such as ER-expressing tumors like those seen in breast and prostate cancers. For this reason, estradiol mustard and other cytostatic-linked estrogens like estramustine phosphate have reduced toxicity relative to non-linked nitrogen mustard cytostatic antineoplastic agents. However, they may stimulate breast tumor growth due to their inherent estrogenic activity and are said to be devoid of major therapeutic efficacy in breast cancer, although estramustine phosphate has been approved for and is used in the treatment of prostate cancer.
Lipoidal estradiol (LE2) is the variety of endogenous C17β long-chain fatty acid esters of estradiol which are formed as metabolites of estradiol. Important examples of these esters include estradiol arachidonate, estradiol lineolate, estradiol oleate, estradiol palmitate, and estradiol stearate. LE2 are estrogens but do not bind to the estrogen receptor, instead acting as prohormones of estradiol. Relative to estradiol, they have far longer-lasting durations of effect due to their much slower rates of metabolism and clearance. It has been hypothesized that LE2 may serve as a store of estrogen for when estradiol levels become low. LE2 are highly lipophilic and hydrophobic and are found in highest concentrations in adipose tissue and other estrogen-sensitive tissues and in low but detectable concentrations in circulation, with none excreted in urine. They have been referred to as the "endogenous counterparts of the synthetic esters of estrogens" like estradiol valerate and estradiol cypionate.

Estramustine is an estrogen and cytostatic antineoplastic agent which was never marketed. It is a carbamate derivative of estradiol and acts in part as a prodrug of estradiol in the body. Estramustine phosphate, the C17β phosphate ester of estramustine and a prodrug of estramustine, estromustine, estradiol, and estrone, is marketed and used in the treatment of prostate cancer.

Nitromifene (INNTooltip International Nonproprietary Name; also as the citrate salt nitromifene citrate (USANTooltip United States Adopted Name), developmental code names CI-628, CN-5518, CN-55945) is a nonsteroidal selective estrogen receptor modulator (SERM) related to triphenylethylenes like tamoxifen that was never marketed. It is a mixture of (E)- and (Z)-isomers that possess similar antiestrogenic activity. The drug was described in 1966. Along with tamoxifen, nafoxidine, and clomifene, it was one of the earliest SERMs.