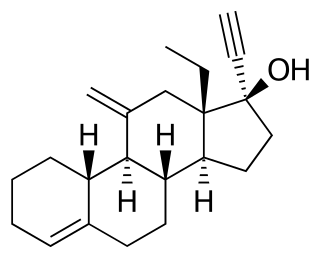
Desogestrel is a progestin medication which is used in birth control pills. It is also used in the treatment of menopausal symptoms in women. The medication is available and used alone or in combination with an estrogen. It is taken by mouth.

Heptabarb, also known as heptabarbitone (BAN) or heptabarbital, is a sedative and hypnotic drug of the barbiturate family. It was used in Europe for the treatment of insomnia from the 1950s onwards, but has since been discontinued.
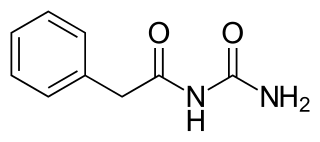
Phenacemide, also known as phenylacetylurea, is an anticonvulsant of the ureide (acetylurea) class. It is a congener and ring-opened analogue of phenytoin, and is structurally related to the barbiturates and to other hydantoins. Phenacemide was introduced in 1949 for the treatment of epilepsy, but was eventually withdrawn due to toxicity.

Etynodiol, or ethynodiol, is a steroidal progestin of the 19-nortestosterone group which was never marketed. A diacylated derivative, etynodiol diacetate, is used as a hormonal contraceptive. Etynodiol is sometimes used as a synonym for etynodiol diacetate.

Norgestrienone, sold under the brand names Ogyline, Planor, and Miniplanor, is a progestin medication which has been used in birth control pills, sometimes in combination with ethinylestradiol. It was developed by Roussel Uclaf and has been registered for use only in France. Under the brand name Planor, it has been marketed in France as 2 mg norgestrienone and 50 μg ethinylestradiol tablets. It is taken by mouth.

Mestranol, sold under the brand names Enovid, Norinyl, and Ortho-Novum among others, is an estrogen medication which has been used in birth control pills, menopausal hormone therapy, and the treatment of menstrual disorders. It is formulated in combination with a progestin and is not available alone. It is taken by mouth.

Minaxolone (CCI-12923) is a neuroactive steroid which was developed as a general anesthetic but was withdrawn before registration due to toxicity seen with long-term administration in rats, and hence was never marketed. It is a positive allosteric modulator of the GABAA receptor, as well as, less potently, a positive allosteric modulator of the glycine receptor.

Aceburic acid (INN), also known as 4-acetoxybutanoic acid or 4-hydroxybutyric acid acetate, is a drug described as an analgesic which was never marketed. It is the acetyl ester of gamma-hydroxybutyrate, and based on its structural relation to GHB, is likely to behave as a prodrug to it.

Oxetorone, as oxetorone fumarate, is a serotonin antagonist, antihistamine, and alpha blocker used as an antimigraine drug. Association with hyperprolactinemia has been described and antidopaminergic actions are also suspected.

Promestriene, also known as estradiol 3-propyl 17β-methyl diether, is a synthetic estrogen which is used topically in a 1% cream formulation for the treatment of vaginal atrophy in women. It is the 3-propyl and 17β-methyl diether of estradiol and does not appear to convert into estradiol in the body. Promestriene is minimally absorbed and appears to have negligible systemic estrogenic effect. The drug has been described as a tropic agent and antiseborrheic. It has not been found to be effective in the treatment of pattern hair loss or other conditions of cutaneous androgenization. Promestriene was first introduced in France in 1974 and has been marketed in 34 countries worldwide. It has been used in millions of women.

Methallenestril, also known as methallenoestril and as methallenestrol, as well as Horeau's acid, is a synthetic nonsteroidal estrogen and a derivative of allenolic acid and allenestrol that was formerly used to treat menstrual issues but is now no longer marketed. It is a seco-analogue of bisdehydrodoisynolic acid, and although methallenestril is potently estrogenic in rats, in humans it is only weakly so in comparison. Vallestril was a brand of methallenestril issued by G. D. Searle & Company in the 1950s. Methallenestril is taken by mouth. By the oral route, a dose of 25 mg methallenestril is approximately equivalent to 1 mg diethylstilbestrol, 4 mg dienestrol, 20 mg hexestrol, 25 mg estrone, 2.5 mg conjugated estrogens, and 0.05 mg ethinylestradiol.
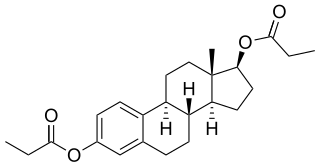
Estradiol dipropionate (EDP), sold under the brand names Agofollin, Di-Ovocylin, and Progynon DP among others, is an estrogen medication which has been used in hormone therapy for menopausal symptoms and low estrogen levels in women and in the treatment of gynecological disorders. It has also been used in feminizing hormone therapy for transgender women and in the treatment of prostate cancer in men. Although widely used in the past, estradiol dipropionate has largely been discontinued and is mostly no longer available today. It appears to remain in use only in Japan, Macedonia, and Australia. Estradiol dipropionate is given by injection into muscle at intervals ranging from once or twice a week to once every week and a half to two weeks.

Formetorex (INN), also known as formetamide or N-formylamphetamine, is a substituted amphetamine described as an anorectic which does not appear to have ever been marketed.
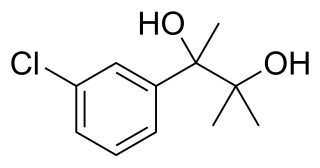
Metaglycodol (INN) is a drug described as a tranquilizer which was never marketed.

Perafensine (INN) is a drug which was investigated as an antidepressant but was never marketed. It has been reported to antagonize the effects of reserpine and to inhibit the reuptake of norepinephrine ; whether it also affects the reuptake of serotonin or dopamine is unclear.

Hexestrol, sold under the brand name Synestrol among others, is a nonsteroidal estrogen which was previously used for estrogen replacement therapy and in the treatment of certain hormone-dependent cancers as well as gynecological disorders but is mostly no longer marketed. It has also been used in the form of esters such as hexestrol diacetate and hexestrol dipropionate. Hexestrol and its esters are taken by mouth, held under the tongue, or via injection into muscle.

Methestrol dipropionate or methoestrol dipropionate, also known as promethestrol dipropionate or promethoestrol dipropionate or as dimethylhexestrol dipropionate, is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol that is or was used clinically. It is the dipropionate form of methestrol, which, in contrast to methestrol dipropionate, was never marketed.

Allenolic acid, or allenoic acid, is a synthetic, nonsteroidal estrogen discovered in 1947 or 1948 that, although studied clinically, was never marketed. It is an open-ring or seco-analogue of steroidal estrogens like estrone and equilenin. The compound was named after Edgar Allen, one of the pioneers in estrogen research. Although described as an estrogen, allenolic acid probably is totally inactive at the receptor, whereas a derivative, allenestrol, is reported to be a potent estrogen. Another derivative of allenolic acid, methallenestril, is also a potent estrogen and, in contrast to allenolic acid and allenestrol, has been marketed.
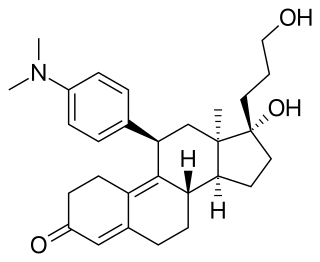
Onapristone is a synthetic and steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering and described in 1984 but was never marketed. It is a silent antagonist of the progesterone receptor (PR), in contrast to the related antiprogestogen mifepristone. Moreover, compared to mifepristone, onapristone has reduced antiglucocorticoid activity, shows little antiandrogenic activity, and has 10- to 30-fold greater potency as an antiprogestogen. The medication was under development for clinical use, for instance in the treatment of breast cancer and as an endometrial contraceptive, but was discontinued during phase III clinical trials in 1995 due to findings that liver function abnormalities developed in a majority patients.

Estradiol benzoate/estradiol dienanthate/testosterone enanthate benzilic acid hydrazone (EB/EDE/TEBH), sold under the brand names Climacteron, Lactimex, Lactostat, and Amenose, is an injectable combination medication of estradiol benzoate (EB), an estrogen, estradiol dienanthate (EDE), an estrogen, and testosterone enanthate benzilic acid hydrazone (TEBH), an androgen/anabolic steroid, which is used in menopausal hormone therapy for peri- and postmenopausal women and to suppress lactation in postpartum women. Clinical studies have assessed this formulation.




















