| |
| Names | |
|---|---|
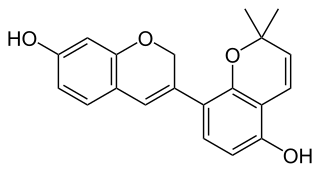
| IUPAC name (3R,13S,17R,18S)-7,13,16,17-Tetrahydroxy-2,2-dimethyl-10-oxapentacyclo[14.2.1.03,12.04,9.013,18]nonadeca-4,6,8,11-tetraen-14-one | |
| Other names (+)-Miroestrol | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| KEGG | |
PubChem CID | |
| |
| |
| Properties | |
| C20H22O6 | |
| Molar mass | 358.385 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Miroestrol is a phytoestrogen, a plant-derived chemical that mimics the biological activity of the hormone estrogen. Miroestrol was first reportedly isolated from the Thai herb Pueraria mirifica in 1960 and thought to be responsible for the supposed rejuvenating properties of the plant. [1] However, more recent studies have suggested that the active ingredient may actually be the closely related chemical compound deoxymiroestrol (shown below), and the reported presence of miroestrol may only have been an artifact of the isolation procedure. [2] When deoxymiroestrol is exposed to the oxygen in air, it is converted to miroestrol.
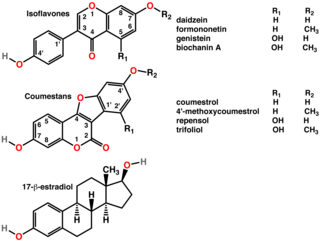
A phytoestrogen is a plant-derived xenoestrogen not generated within the endocrine system, but consumed by eating phytoestrogenic plants. Also called a "dietary estrogen", it is a diverse group of naturally occurring nonsteroidal plant compounds that, because of its structural similarity with estradiol (17-β-estradiol), have the ability to cause estrogenic and/or antiestrogenic effects. Phytoestrogens are not essential nutrients because their absence from the diet does not cause a disease, nor are they known to participate in any normal biological function.

Estrogen, or oestrogen, is the primary female sex hormone. It is responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens in females that have estrogenic hormonal activity: estrone, estradiol, and estriol. The estrane steroid estradiol is the most potent and prevalent of these.
Pueraria mirifica, also known as กวาวเครือ Kwao Krua, is a plant found in northern and north eastern Thailand and Myanmar.

A comparative study of the estrogenic properties of phytoestrogens found that both deoxymiroestrol and miroestrol were comparable in activity in vitro to other known phytoestrogens such as coumestrol as 17β-oestradiol agonists. [3] Because of their estrogenic activities, miroestrol, deoxymiroestrol, and other related compounds have been the targets of scientific research including total synthesis. [4] [5]

In vitro studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from in vitro experiments may not fully or accurately predict the effects on a whole organism. In contrast to in vitro experiments, in vivo studies are those conducted in animals, including humans, and whole plants.

Coumestrol is a natural organic compound in the class of phytochemicals known as coumestans. Coumestrol was first identified as a compound with estrogenic properties by E. M. Bickoff in ladino clover and alfalfa in 1957. It has garnered research interest because of its estrogenic activity and prevalence in some foods, including soybeans, brussels sprouts, spinach and a variety of legumes. The highest concentrations of coumestrol are found in clover, Kala Chana, a type of chick pea, and Alfalfa sprouts.
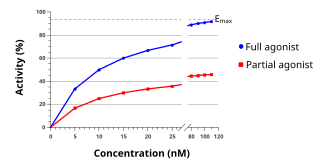
An agonist is a chemical that binds to a receptor and activates the receptor to produce a biological response. Whereas an agonist causes an action, an antagonist blocks the action of the agonist, and an inverse agonist causes an action opposite to that of the agonist.
Extracts of Pueraria mirifica reportedly containing miroestrol are marketed as dietary supplements intended to lead to breast enhancement in women. However, there is a lack of conclusive evidence for such claims. The Federal Trade Commission has taken legal action against marketers for these unproven claims. [6]

The Federal Trade Commission (FTC) is an independent agency of the United States government, established in 1914 by the Federal Trade Commission Act. Its principal mission is the promotion of consumer protection and the elimination and prevention of anticompetitive business practices, such as coercive monopoly. It is headquartered in the Federal Trade Commission Building in Washington, D.C.