
Diethylstilbestrol (DES), also known as stilbestrol or stilboestrol, is a nonsteroidal estrogen medication, which is presently rarely used. In the past, it was widely used for a variety of indications, including pregnancy support for those with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency, treatment of prostate cancer and breast cancer, and other uses. By 2007, it was only used in the treatment of prostate cancer and breast cancer. In 2011, Hoover and colleagues reported on adverse health outcomes linked to DES including infertility, miscarriage, ectopic pregnancy, preeclampsia, preterm birth, stillbirth, infant death, menopause prior to age 45, breast cancer, cervical cancer, and vaginal cancer. While most commonly taken by mouth, DES was available for use by other routes as well, for instance, vaginal, topical, and by injection.
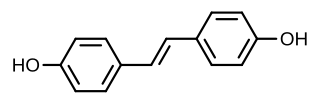
Stilbestrol, or stilboestrol, also known as 4,4'-dihydroxystilbene or 4,4'-stilbenediol, is a stilbenoid nonsteroidal estrogen and the parent compound of a group of more potent nonsteroidal estrogen derivatives that includes, most notably, diethylstilbestrol (DES). The term "stilbestrol" is often used incorrectly to refer to DES, but they are not the same compound.

Fosfestrol, sold under the brand name Honvan and also known as diethylstilbestrol diphosphate (DESDP), is an estrogen medication which is used in the treatment of prostate cancer in men. It is given by slow intravenous infusion once per day to once per week or by mouth once per day.

Chlorotrianisene (CTA), also known as tri-p-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal symptoms and estrogen deficiency in women and prostate cancer in men, among other indications, but has since been discontinued and is now no longer available. It is taken by mouth.

Methallenestril, also known as methallenoestril and as methallenestrol, as well as Horeau's acid, is a synthetic nonsteroidal estrogen and a derivative of allenolic acid and allenestrol that was formerly used to treat menstrual issues but is now no longer marketed. It is a seco-analogue of bisdehydrodoisynolic acid, and although methallenestril is potently estrogenic in rats, in humans it is only weakly so in comparison. Vallestril was a brand of methallenestril issued by G. D. Searle & Company in the 1950s. Methallenestril is taken by mouth. By the oral route, a dose of 25 mg methallenestril is approximately equivalent to 1 mg diethylstilbestrol, 4 mg dienestrol, 20 mg hexestrol, 25 mg estrone, 2.5 mg conjugated estrogens, and 0.05 mg ethinylestradiol.

Benzestrol is a synthetic nonsteroidal estrogen of the stilbestrol group which was formerly used medically but has since been discontinued. The stilbestrol estrogens, the best-known of which is diethylstilbestrol (DES) were used extensively in the mid-1900s and were finally banned by the FDA due to them causing tumors in the children of women who used them.

Paroxypropione, also known as paraoxypropiophenone, is a synthetic nonsteroidal estrogen which has been used medically as an antigonadotropin in Spain and Italy but appears to no longer be marketed. It was first synthesized in 1902. The antigonadotropic properties of the drug were discovered in 1951 and it entered clinical use shortly thereafter.

A nonsteroidal estrogen is an estrogen with a nonsteroidal chemical structure. The most well-known example is the stilbestrol estrogen diethylstilbestrol (DES). Although nonsteroidal estrogens formerly had an important place in medicine, they have gradually fallen out of favor following the discovery of toxicities associated with high-dose DES starting in the early 1970s, and are now almost never used. On the other hand, virtually all selective estrogen receptor modulators (SERMs) are nonsteroidal, with triphenylethylenes like tamoxifen and clomifene having been derived from DES, and these drugs remain widely used in medicine for the treatment of breast cancer among other indications. In addition to pharmaceutical drugs, many xenoestrogens, including phytoestrogens, mycoestrogens, and synthetic endocrine disruptors like bisphenol A, are nonsteroidal substances with estrogenic activity.
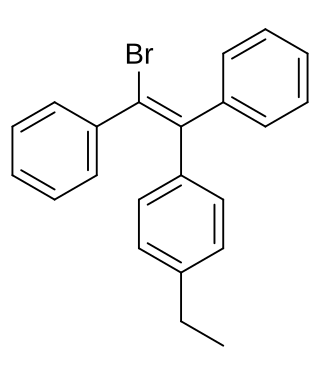
Broparestrol, also known as α-bromo-α,β-diphenyl-β-p-ethylphenylethylene (BDPE), is a synthetic, nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that has been used in Europe as a dermatological agent and for the treatment of breast cancer. The drug is described as slightly estrogenic and potently antiestrogenic, and inhibits mammary gland development and suppresses prolactin levels in animals. It is structurally related to clomifene and diethylstilbestrol. Broparestrol is a mixture of E- and Z- isomers, both of which are active, and are similarly antiestrogenic but, unlike broparestrol, were never marketed.

Hexestrol, sold under the brand name Synestrol among others, is a nonsteroidal estrogen which was previously used for estrogen replacement therapy and in the treatment of certain hormone-dependent cancers as well as gynecological disorders but is mostly no longer marketed. It has also been used in the form of esters such as hexestrol diacetate and hexestrol dipropionate. Hexestrol and its esters are taken by mouth, held under the tongue, or via injection into muscle.

Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity. Its estrogenic effects were discovered in 1937. TPE was derived from structural modification of the more potent estrogen diethylstilbestrol, which is a member of the stilbestrol group of nonsteroidal estrogens.

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.
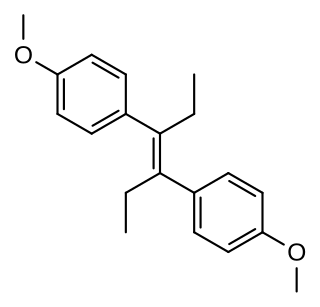
Dimestrol, also known as dianisylhexene, 4,4'-dimethoxy-α,α'-diethylstilbene, diethylstilbestrol dimethyl ether, and dimethoxydiethylstilbestrol, is a synthetic nonsteroidal estrogen of the stilbestrol group which is related to diethylstilbestrol. It has been used clinically as a hormonal therapy in cases of delayed female puberty, hypogonadism, menopausal, and postmenopausal symptoms. It is known to induce the development of female secondary sexual characteristics in the case of female delayed puberty or hypogonadism. The drug has also been used as a growth promoter in livestock.

Furostilbestrol (INN), also known as diethylstilbestrol di(2-furoate) or simply as diethylstilbestrol difuroate, is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol, that was never marketed. It is an ester of diethylstilbestrol and was described in the literature in 1952.

Dianol is a synthetic, nonsteroidal estrogen that was never marketed. It is a dimer and impurity of anol, and was, along with hexestrol, involved in erroneous findings of highly potent estrogenic activity with anol. Although a potent estrogen, it requires a dose of 100 μg to show activity, whereas hexestrol shows activity with a mere dose of 0.2 μg.
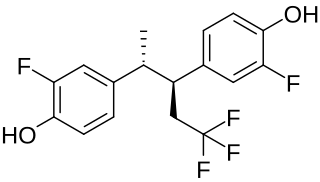
Pentafluranol is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol that was developed for the treatment of benign prostatic hyperplasia never marketed. It was described in the medical literature in 1974.
Diethylstilbestrol dilaurate is a synthetic, nonsteroidal estrogen of the stilbestrol group and an ester of diethylstilbestrol (DES) that was previously marketed but is now no longer available. It was formulated and used as a micronized topical medication to treat acne in adolescent boys and young men. The drug was marketed as early as 1951.
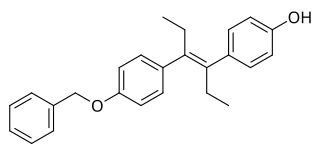
Diethylstilbestrol monobenzyl ether, also known as benzelstilbestrol, is a synthetic, nonsteroidal estrogen of the stilbestrol group and an ether of diethylstilbestrol (DES) that is described as a pituitary gland inhibitor (antigonadotropin) and was formerly marketed but is now no longer available. It was first synthesized by Wallace & Tiernan Company in 1952, and was described by them as having only weak estrogenic activity. The drug was used to treat gynecological conditions and infertility in women.
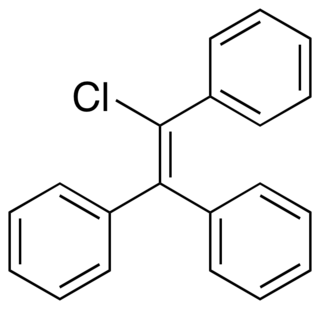
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.


















