Werner Emmanuel Bachmann was an American chemist. Bachmann was born in Detroit, Michigan where he studied chemistry and chemical engineering at Wayne State University and later at the University of Michigan in Ann Arbor nearby. He completed his doctorate under Moses Gomberg and spent the rest of his academic career at the University of Michigan.
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes it from semisynthesis. Syntheses may sometimes conclude at a precursor with further known synthetic pathways to a target molecule, in which case it is known as a formal synthesis. Total synthesis target molecules can be natural products, medicinally-important active ingredients, known intermediates, or molecules of theoretical interest. Total synthesis targets can also be organometallic or inorganic, though these are rarely encountered. Total synthesis projects often require a wide diversity of reactions and reagents, and subsequently requires broad chemical knowledge and training to be successful.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.

A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of organolithium reagent. The reaction was discovered by Robert H. Shapiro in 1967. The Shapiro reaction was used in the Nicolaou Taxol total synthesis. This reaction is very similar to the Bamford–Stevens reaction, which also involves the basic decomposition of tosyl hydrazones.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.

The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of taxol. Taxol is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the pacific yew.

The Fujimoto–Belleau reaction is a chemical reaction that forms cyclic α-substituted α,β-unsaturated ketones from enol lactones. The reaction was discovered in 1951 by George I. Fujimoto and Bernard Belleau. Belleau used this reaction to synthesize 1-methyl-3-keto-1,2,3,9,10,10a-hexahydrophenanthrene from a ketoacid starting material and Fujimoto demonstrated that steroids could be synthesized from naturally occurring lactone species using this method as well.
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.

Diosgenin, a phytosteroid sapogenin, is the product of hydrolysis by acids, strong bases, or enzymes of saponins, extracted from the tubers of Dioscorea wild yam species, such as the Kokoro. It is also present in smaller amounts in a number of other species. The sugar-free (aglycone) product of such hydrolysis, diosgenin is used for the commercial synthesis of cortisone, pregnenolone, progesterone, and other steroid products.
The total synthesis of quinine, a naturally-occurring antimalarial drug, was developed over a 150-year period. The development of synthetic quinine is considered a milestone in organic chemistry although it has never been produced industrially as a substitute for natural occurring quinine. The subject has also been attended with some controversy: Gilbert Stork published the first stereoselective total synthesis of quinine in 2001, meanwhile shedding doubt on the earlier claim by Robert Burns Woodward and William Doering in 1944, claiming that the final steps required to convert their last synthetic intermediate, quinotoxine, into quinine would not have worked had Woodward and Doering attempted to perform the experiment. A 2001 editorial published in Chemical & Engineering News sided with Stork, but the controversy was eventually laid to rest once and for all when Robert Williams and coworkers successfully repeated Woodward's proposed conversion of quinotoxine to quinine in 2007.
The Marker degradation is a three-step synthetic route in steroid chemistry developed by American chemist Russell Earl Marker in 1938–1940. It is used for the production of cortisone and mammalian sex hormones from plant steroids, and established Mexico as a world center for steroid production in the years immediately after World War II. The discovery of the Marker degradation allowed the production of substantial quantities of steroid hormones for the first time, and was fundamental in the development of the contraceptive pill and corticosteroid anti-inflammatory drugs. In 1999, the American Chemical Society and the Sociedad Química de México named the route as an International Historic Chemical Landmark.
The Bucherer–Bergs reaction is the chemical reaction of carbonyl compounds or cyanohydrins with ammonium carbonate and potassium cyanide to give hydantoins. The reaction is named after Hans Theodor Bucherer.

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field.

Endiandric acid C, isolated from the tree Endiandra introrsa, is a well characterized chemical compound. Endiadric acid C is reported to have better antibiotic activity than ampicillin.
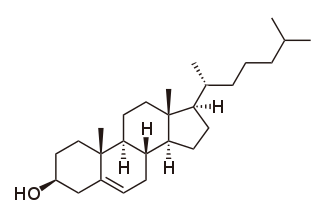
Cholesterol total synthesis in chemistry describes the total synthesis of the complex biomolecule cholesterol and is considered a great scientific achievement. The research group of Robert Robinson with John Cornforth published their synthesis in 1951 and that of Robert Burns Woodward with Franz Sondheimer in 1952. Both groups competed for the first publication since 1950 with Robinson having started in 1932 and Woodward in 1949. According to historian Greg Mulheirn the Robinson effort was hampered by his micromanagement style of leadership and the Woodward effort was greatly facilitated by his good relationships with chemical industry. Around 1949 steroids like cortisone were produced from natural resources but expensive. Chemical companies Merck & Co. and Monsanto saw commercial opportunities for steroid synthesis and not only funded Woodward but also provided him with large quantities of certain chemical intermediates from pilot plants. Hard work also helped the Woodward effort: one of the intermediate compounds was named Christmasterone as it was synthesized on Christmas Day 1950 by Sondheimer.
Alfred Lawrence Wilds was a professor emeritus of chemistry at the University of Wisconsin in Madison.
Biomimetic synthesis is an area of organic chemical synthesis that is specifically biologically inspired. The term encompasses both the testing of a "biogenetic hypothesis" through execution of a series of reactions designed to parallel the proposed biosynthesis, as well as programs of study where a synthetic reaction or reactions aimed at a desired synthetic goal are designed to mimic one or more known enzymic transformations of an established biosynthetic pathway. The earliest generally cited example of a biomimetic synthesis is Sir Robert Robinson's organic synthesis of the alkaloid tropinone.

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

Bisdehydrodoisynolic acid (BDDA), as the (Z)-isomer ( -BDDA), is a synthetic, nonsteroidal estrogen related to doisynolic acid that was never marketed. It is one of the most potent estrogens known, although it has more recently been characterized as a selective estrogen receptor modulator (SERM). BDDA and other doisynolic acid derivatives display relatively low affinity accompanied by disproportionately high estrogenic potency in vivo, which was eventually determined to be due to transformation into metabolites with greater estrogenic activity. The drug was discovered in 1947 as a degradation product of the reaction of equilenin or dihydroequilenin with potassium hydroxide. It is the seco-analogue of equilenin, while doisynolic acid is the seco-analogue of estrone. These compounds, along with diethylstilbestrol, can be considered to be open-ring analogues of estradiol. The methyl ether of BDDA, doisynoestrol, is also an estrogen, and in contrast to BDDA, has been marketed.











