
Kudzu, also called Japanese arrowroot or Chinese arrowroot, is a group of climbing, coiling, and trailing deciduous perennial vines native to much of East Asia, Southeast Asia, and some Pacific islands, but invasive in many parts of the world, primarily North America.

Pueraria montana is a species of plant in the botanical family Fabaceae. At least three sub-species are known. It is closely related to other species in the genus Pueraria and the common name kudzu is used for all of these species and hybrids between them. The morphological differences between them are subtle, they can breed with each other, and it appears that introduced kudzu populations in the United States have ancestry from more than one of the species.

Pueraria is a genus of 15–20 species of legumes native to south, east, and southeast Asia and to New Guinea and northern Australia. The best known member is kudzu, also called Japanese arrowroot. The genus is named after 19th century Swiss botanist Marc Nicolas Puerari.

Equol (4',7-isoflavandiol) is an isoflavandiol estrogen metabolized from daidzein, a type of isoflavone found in soybeans and other plant sources, by bacterial flora in the intestines. While endogenous estrogenic hormones such as estradiol are steroids, equol is a nonsteroidal estrogen. Only about 30–50% of people have intestinal bacteria that make equol.

Imperatorin is a furocoumarin and a phytochemical that has been isolated from Urena lobata L. (Malvaceae), Angelica archangelica, Angelica dahurica, Glehnia littoralis, Saposhnikovia divaricata, Cnidium monnieri, Incarvillea younghusbandii, and Zanthoxylum americanum mill. It is biosynthesized from umbelliferone, a coumarin derivative.
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones occur in many plant species, but are especially high in soybeans.

Genistein (C15H10O5) is a naturally occurring compound that structurally belongs to a class of compounds known as isoflavones. It is described as an angiogenesis inhibitor and a phytoestrogen.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility.
The enzyme 2-hydroxyisoflavanone dehydratase (EC 4.2.1.105) catalyzes the chemical reaction
A self-microemulsifying drug delivery system (SMEDDS) is a drug delivery system that uses a microemulsion achieved by chemical rather than mechanical means. That is, by an intrinsic property of the drug formulation, rather than by special mixing and handling. It employs the familiar ouzo effect displayed by anethole in many anise-flavored liquors. Microemulsions have significant potential for use in drug delivery, and SMEDDS are the best of these systems identified to date. SMEDDS are of particular value in increasing the absorption of lipophilic drugs taken by mouth.

Puerarin, one of several known isoflavones, is found in a number of plants and herbs, such as the root of the kudzu plant

Ononin is an isoflavone glycoside, the 7-O-β-D-glucopyranoside of formononetin, which in turn is the 4'-O-methoxy derivative of the parent isoflavone daidzein.

The flavanonols are a class of flavonoids that use the 3-hydroxy-2,3-dihydro-2-phenylchromen-4-one backbone.
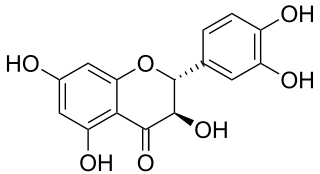
Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols. It is extracted from plants such as Siberian larch and milk thistle.

Huáng bǎi, huáng bó or huáng bò is one of the fifty fundamental herbs of traditional Chinese medicine. Known also as Cortex Phellodendri, it is the bark of one of two species of Phellodendron tree: Phellodendron amurense or Phellodendron chinense.

Barbigerone is one of a few pyranoisoflavones among several groups of isoflavones. It was first isolated from the seed of a leguminous plant Tephrosia barbigera; hence the name "barbigerone". Members of the genus Millettia are now known to be rich in barbigerone, including M. dielsiena, M. ferruginea, M. usaramensis, and M. pachycarpa. It has also been isolated from the medicinal plant Sarcolobus globosus. Barbigerone from S. globosus is validated to have significant antioxidant property. Barbigerone exhibits profound antiplasmodial activity against the malarial parasite Plasmodium falciparum. It is also demonstrated that it has anti-cancer potential as it causes apoptosis of murine lung-cancer cells.

Pterocarpans are derivatives of isoflavonoids found in the family Fabaceae. It is a group of compounds which can be described as benzo-pyrano-furano-benzenes which can be formed by coupling of the B ring to the 4-one position.

Fraxetin is an O-methylated coumarin. It can be found in Fraxinus rhynchophylla and seeds of Datura stramonium. Fraxin is a glucoside of fraxetin.

Mirificin, also known as daidzein 8-C-(6-apiofuranosylglucoside), is an isoflavone that is found in Pueraria mirifica and Pueraria lobata. It has estrogenic activity and hence is a phytoestrogen.

Securinine is an alkaloid found in Securinega suffruticosa and Phyllanthus niruri.

















