Monoamine oxidases (MAO) are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases.

Disulfiram is a medication used to support the treatment of chronic alcoholism by producing an acute sensitivity to ethanol. Disulfiram works by inhibiting the enzyme aldehyde dehydrogenase, causing many of the effects of a hangover to be felt immediately following alcohol consumption. Disulfiram plus alcohol, even small amounts, produces flushing, throbbing in the head and neck, a throbbing headache, respiratory difficulty, nausea, copious vomiting, sweating, thirst, chest pain, palpitation, dyspnea, hyperventilation, fast heart rate, low blood pressure, fainting, marked uneasiness, weakness, vertigo, blurred vision, and confusion. In severe reactions there may be respiratory depression, cardiovascular collapse, abnormal heart rhythms, heart attack, acute congestive heart failure, unconsciousness, convulsions, and death.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specifically, a neurotoxin or neurotoxicant– alters the normal activity of the nervous system in such a way as to cause permanent or reversible damage to nervous tissue. This can eventually disrupt or even kill neurons, which are cells that transmit and process signals in the brain and other parts of the nervous system. Neurotoxicity can result from organ transplants, radiation treatment, certain drug therapies, recreational drug use, exposure to heavy metals, bites from certain species of venomous snakes, pesticides, certain industrial cleaning solvents, fuels and certain naturally occurring substances. Symptoms may appear immediately after exposure or be delayed. They may include limb weakness or numbness, loss of memory, vision, and/or intellect, uncontrollable obsessive and/or compulsive behaviors, delusions, headache, cognitive and behavioral problems and sexual dysfunction. Chronic mold exposure in homes can lead to neurotoxicity which may not appear for months to years of exposure. All symptoms listed above are consistent with mold mycotoxin accumulation.

Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl, Zelapar, and Emsam among others, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder. It has also been studied for a variety of other indications, but has not been formally approved for any other use. The medication in the form licensed for depression has modest effectiveness for this condition that is similar to that of other antidepressants. Selegiline is provided as a swallowed tablet or capsule or an orally disintegrating tablet (ODT) for Parkinson's disease and as a patch applied to skin for depression.

Dopaminergic means "related to dopamine", a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.
Aldehyde dehydrogenases are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes to carboxylic acids. The oxygen comes from a water molecule. To date, nineteen ALDH genes have been identified within the human genome. These genes participate in a wide variety of biological processes including the detoxification of exogenously and endogenously generated aldehydes.

Rasagiline, sold under the brand name Azilect among others, is a medication which is used in the treatment of Parkinson's disease. It is used as a monotherapy to treat symptoms in early Parkinson's disease or as an adjunct therapy in more advanced cases. The drug is taken by mouth.

Pargyline, sold under the brand name Eutonyl among others, is a monoamine oxidase inhibitor (MAOI) medication which has been used to treat hypertension but is no longer marketed. It has also been studied as an antidepressant, but was never licensed for use in the treatment of depression. The drug is taken by mouth.
PC12 is a cell line derived from a pheochromocytoma of the rat adrenal medulla, that have an embryonic origin from the neural crest that has a mixture of neuroblastic cells and eosinophilic cells.

(–)-Benzofuranylpropylaminopentane is an experimental drug related to selegiline which acts as a monoaminergic activity enhancer (MAE). It is orally active in animals.

Monoamine oxidase B, also known as MAO-B, is an enzyme that in humans is encoded by the MAOB gene.

A disulfiram-like drug is a drug that causes an adverse reaction to alcohol leading to nausea, vomiting, flushing, dizziness, throbbing headache, chest and abdominal discomfort, and general hangover-like symptoms among others. These effects are caused by accumulation of acetaldehyde, a major but toxic metabolite of alcohol formed by the enzyme alcohol dehydrogenase. The reaction has been variously termed a disulfiram-like reaction, alcohol intolerance, and acetaldehyde syndrome.

Animal models of Parkinson's disease are essential in the research field and widely used to study Parkinson's disease. Parkinson's disease is a neurodegenerative disorder, characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The loss of the dopamine neurons in the brain, results in motor dysfunction, ultimately causing the four cardinal symptoms of PD: tremor, rigidity, postural instability, and bradykinesia. It is the second most prevalent neurodegenerative disease, following Alzheimer's disease. It is estimated that nearly one million people could be living with PD in the United States.
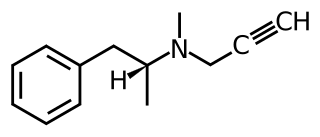
Monoaminergic activity enhancers (MAE), also known as catecholaminergic/serotonergic activity enhancers (CAE/SAE), are a class of compounds that enhance the action potential-evoked release of monoamine neurotransmitters in the nervous system. MAEs are distinct from monoamine releasing agents (MRAs) like amphetamine and fenfluramine in that they do not induce the release of monoamines from synaptic vesicles but rather potentiate only nerve impulse propagation-mediated monoamine release. That is, MAEs increase the amounts of monoamine neurotransmitters released by neurons per electrical impulse.

Desmethylselegiline (DMS), also known as norselegiline or as N-propargyl-L-amphetamine, is an active metabolite of selegiline, a medication used in the treatment of Parkinson's disease and depression.

The pharmacology of selegiline is the study of the pharmacodynamic and pharmacokinetic properties of the antiparkinsonian and antidepressant selegiline (L-deprenyl). Selegiline is available in a few different forms, including oral tablets and capsules, orally disintegrating tablets (ODTs), and transdermal patches. These forms have differing pharmacological properties.

A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine. Examples of monoamine neurotoxins include the serotonergic neurotoxins para-chloroamphetamine (PCA), methylenedioxymethamphetamine (MDMA), and 5,7-dihydroxytryptamine (5,7-DHT); the dopaminergic neurotoxins oxidopamine (6-hydroxydopamine), MPTP, and methamphetamine; and the noradrenergic neurotoxins oxidopamine and DSP-4. Dopaminergic neurotoxins can induce a Parkinson's disease-like condition in animals and humans. Serotonergic neurotoxins have been associated with cognitive and memory deficits and psychiatric changes.

5-Hydroxyindoleacetaldehyde (5-HIAL), also known as 5-hydroxytryptaldehyde or as serotonin aldehyde, is an inactive metabolite and metabolic intermediate of the monoamine neurotransmitter serotonin.

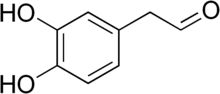
3,4-Dihydroxyphenylglycolaldehyde (DOPEGAL), also known as 3,4-dihydroxymandelaldehyde (DHMAL) as well as norepinephrine aldehyde or epinephrine aldehyde, is a metabolite of the monoamine neurotransmitters norepinephrine and epinephrine. DOPEGAL is a noradrenergic neurotoxin.