
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is an organic compound. It is classified as a tetrahydropyridine. It is of interest as a precursor to the monoaminergic neurotoxin MPP+, which causes permanent symptoms of Parkinson's disease by destroying dopaminergic neurons in the substantia nigra of the brain. It has been used to study disease models in various animals.
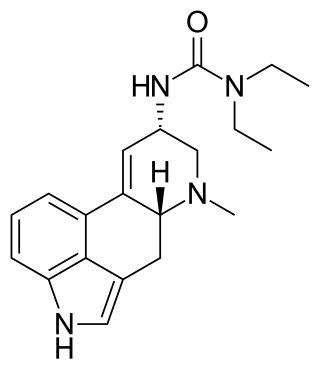
Lisuride, sold under the brand name Dopergin among others, is a monoaminergic medication of the ergoline class which is used in the treatment of Parkinson's disease, migraine, and high prolactin levels. It is taken by mouth.

DSP-4, or N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine, is a monoaminergic neurotoxin selective for noradrenergic neurons, capable of crossing the blood–brain barrier.

(–)-Benzofuranylpropylaminopentane is an experimental drug related to selegiline which acts as a monoaminergic activity enhancer (MAE). It is orally active in animals.
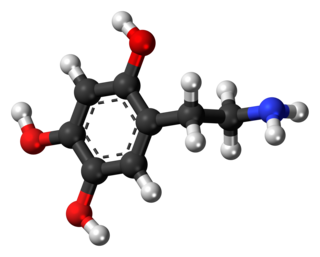
Oxidopamine, also known as 6-hydroxydopamine (6-OHDA) or 2,4,5-trihydroxyphenethylamine, is a synthetic monoaminergic neurotoxin used by researchers to selectively destroy dopaminergic and noradrenergic neurons in the brain.

5,7-Dihydroxytryptamine (5,7-DHT) is a monoaminergic neurotoxin used in scientific research to decrease concentrations of serotonin in the brain. The mechanism behind this effect is not well understood, but it is speculated to selectively destroy serotonergic neurons, in a manner similar to the dopaminergic neurotoxicity of 6-hydroxydopamine (6-OHDA). What is known is that this compound is in fact not selective in depleting serotonin content, but also depletes norepinephrine. To selectively deplete serotonin stores, it is commonly administered in conjunction with desmethylimipramine (desipramine), which inhibits the norepinephrine transporter.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a substituted amphetamine and monoamine releaser similar to MDMA, but with substantially higher activity as a monoaminergic neurotoxin, thought to be due to the unrestrained release of both serotonin and dopamine by a metabolite. It is used as a neurotoxin by neurobiologists to selectively kill serotonergic neurons for research purposes, in the same way that 6-hydroxydopamine is used to kill dopaminergic neurons.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.

Amiflamine (FLA-336) is a reversible inhibitor of monoamine oxidase A (MAO-A), thereby being a RIMA, and, to a lesser extent, semicarbazide-sensitive amine oxidase (SSAO), as well as a serotonin releasing agent (SRA). It is a derivative of the phenethylamine and amphetamine chemical classes. The (+)-enantiomer is the active stereoisomer.
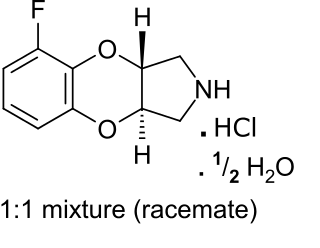
Fluparoxan is a potent α2-adrenergic receptor antagonist with excellent selectivity for this receptor over the α1-adrenergic receptor (2,630-fold), and is the only well-studied α2-adrenergic receptor antagonist in its structural family which does not antagonize any variant of the imidazoline receptor. It was shown to possess central α2-adrenoceptor antagonist activity after oral doses in man and was patented as an antidepressant by Glaxo in the early 1980s, but its development was discontinued when the compound failed to show a clear clinical advantage over existing therapies.
Noradrenergic cell groups refers to collections of neurons in the central nervous system that have been demonstrated by histochemical fluorescence to contain the neurotransmitter norepinephrine (noradrenalin). They are named

A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine.

HPP+, also known as haloperidol pyridinium, is a monoaminergic neurotoxin and a metabolite of haloperidol.

5,6-Dihydroxytryptamine (5,6-DHT) is a monoaminergic neurotoxin and tryptamine derivative related to serotonin (5-hydroxytryptamine) and 5,7-dihydroxytryptamine (5,7-DHT). It is a relatively selective serotonergic neurotoxin, but also acts as a dopaminergic and noradrenergic neurotoxin at higher doses. In addition, it produces widespread generalized toxicity at higher doses. Its selective serotonergic neurotoxicity is due to its high affinity for the serotonin transporter (SERT). Because of its SERT affinity, 5,6-DHT has activity as a serotonin reuptake inhibitor.

6-Hydroxydopa is a catecholaminergic neurotoxin that damages noradrenergic and dopaminergic neurons and is used in scientific research. It is a precursor and prodrug of 6-hydroxydopamine (6-OHDA). The drug is a derivative of levodopa (L-DOPA). It has certain advantages over 6-OHDA, such as the ability to cross the blood–brain barrier into the central nervous system and hence the ability to be administered systemically rather than directly into the brain. 6-OH-DOPA was first described in the scientific literature by 1969.

ODMA is a bioisosteric analogue of 3,4-methylenedioxy-N-methylamphetamine (MDMA) which was developed in an attempt to create an improved MDMA alternative for potential clinical use. It is the analogue of MDMA in which the 1,3-benzodioxole ring has been replaced with a 2,1,3-benzoxadiazole ring. TDMA and SeDMA are closely related analogues. ODMA, TDMA, and SeDMA are releasing agents of serotonin, norepinephrine, and dopamine similarly to MDMA. However, they are less potent and efficacious in activating the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors than MDMA and show differing and potentially improved metabolic and pharmacokinetic properties in comparison. ODMA, TDMA, and SeDMA were first described in the scientific literature in June 2024.

TDMA is a bioisosteric analogue of 3,4-methylenedioxy-N-methylamphetamine (MDMA) which was developed in an attempt to create an improved MDMA alternative for potential clinical use. It is the analogue of MDMA in which the 1,3-benzodioxole ring has been replaced with a 2,1,3-benzothiadiazole ring. ODMA and SeDMA are closely related analogues. ODMA, TDMA, and SeDMA are releasing agents of serotonin, norepinephrine, and dopamine similarly to MDMA. However, they are less potent and efficacious in activating the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors than MDMA and show differing and potentially improved metabolic and pharmacokinetic properties in comparison. ODMA, TDMA, and SeDMA were first described in the scientific literature in June 2024.

SeDMA is a bioisosteric analogue of 3,4-methylenedioxy-N-methylamphetamine (MDMA) which was developed in an attempt to create an improved MDMA alternative for potential clinical use. It is the analogue of MDMA in which the 1,3-benzodioxole ring has been replaced with a 2,1,3-benzoselenadiazole ring. ODMA and TDMA are closely related analogues. ODMA, TDMA, and SeDMA are releasing agents of serotonin, norepinephrine, and dopamine similarly to MDMA. However, they are less potent and efficacious in activating the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors than MDMA and show differing and potentially improved metabolic and pharmacokinetic properties in comparison. ODMA, TDMA, and SeDMA were first described in the scientific literature in June 2024.

2′-NH2-MPTP, also known as 2′-amino-MPTP, is a monoaminergic neurotoxin that was derived from MPTP and is used in scientific research to lesion brain monoaminergic systems in animals. Whereas MPTP is a selective dopaminergic neurotoxin, 2′-NH2-MPTP is a specific serotonergic and noradrenergic neurotoxin that doesn't affect dopaminergic neurons. 2′-NH2-MPTP is transported by the serotonin transporter (SERT) into serotonergic neurons and by the norepinephrine transporter (NET) into noradrenergic neurons, and its serotonergic and noradrenergic neurotoxicity is dependent on this transport by the SERT and NET, respectively. 2′-NH2-MPTP was first described in the scientific literature by 1993.

HPTP is a monoaminergic neurotoxin related to MPTP. It is the dehydration product of haloperidol. The agent is specifically a dopaminergic and serotonergic neurotoxin. HPTP is a prodrug of HPP+, which mediates its monoaminergic neurotoxicity. This is analogous to how MPP+ mediates the neurotoxicity of MPTP. Other related compounds include RHPTP and RHPP+.



















