Dipteryx odorata is a species of flowering tree in the pea family, Fabaceae. The tree is native to Northern South America and is semi-deciduous. Its seeds are known as tonka beans. They are black and wrinkled and have a smooth, brown interior. They have a strong fragrance similar to sweet woodruff due to their high content of coumarin.

Genistein (C15H10O5) is a naturally occurring compound that structurally belongs to a class of compounds known as isoflavones. It is described as an angiogenesis inhibitor and a phytoestrogen.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility.

Honokiol is a lignan isolated from the bark, seed cones, and leaves of trees belonging to the genus Magnolia. It has been identified as one of the chemical compounds in some traditional eastern herbal medicines along with magnolol, 4-O-methylhonokiol, and obovatol.

Maackia amurensis, commonly known as the Amur maackia, is a species of tree in the family Fabaceae that can grow 15 metres (49 ft) tall. The species epithet and common names are from the Amur River region, where the tree originated; it occurs in northeastern China, Korea, and Russia.

Wolfiporia extensa, commonly known as hoelen, poria, tuckahoe, China root, fu ling, or matsuhodo, is a fungus in the family Polyporaceae. It is a wood-decay fungus but has a subterranean growth habit. It is notable in the development of a large, long-lasting underground sclerotium that resembles a small coconut. This sclerotium, known as Tuckahoe or fu-ling, is not the same as the true tuckahoe used as Indian bread by Native Americans, which is the arrow arum, Peltandra virginica, a flowering tuberous plant in the arum family.

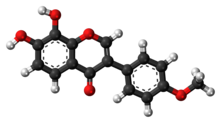
Formononetin is an O-methylated isoflavone.

Prenylated flavonoids or prenylflavonoids are a sub-class of flavonoids. They are widely distributed throughout the plant kingdom. Some are known to have phytoestrogenic or antioxidant properties. They are given in the list of adaptogens in herbalism. Chemically they have a prenyl group attached to their flavonoid backbone. It is usually assumed that the addition of hydrophobic prenyl groups facilitate attachment to cell membranes. Prenylation may increase the potential activity of its original flavonoid.
The Guanling Formation is a Middle Triassic geologic formation in southwestern China.
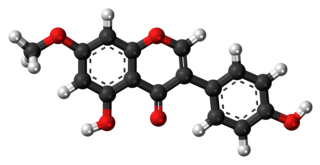
Prunetin is an O-methylated isoflavone, a type of flavonoid. It has been isolated for the first time by Finnemore in 1910 in the bark of Prunus emarginata. Prunetin isolated from pea roots can act as an attractant for Aphanomyces euteiches zoospores. It is also an allosteric inhibitor of human liver aldehyde dehydrogenase.

Alpinumisoflavone is a pyranoisoflavone, a type of isoflavone. It can be found in the bark of Rinorea welwitschii. It can also be found in the molluscicide plant Millettia thonningii and is thought to be an antischistosomal agent since it has been shown to kill the snails which transmit the schistosomiasis and also the larvae of the parasite itself.

Derris taiwaniana is a perennial climbing shrub belonging to the genus Derris. It is known by several synonyms, including Millettia pachycarpa and M. taiwaniana. It is widely used in traditional practices, such as for poisoning fish, agricultural pesticide, blood tonic, and treatments of cancer and infertility. The bark fiber is used for making strong ropes.

Barbigerone is one of a few pyranoisoflavones among several groups of isoflavones. It was first isolated from the seed of a leguminous plant Tephrosia barbigera; hence the name "barbigerone". Members of the genus Millettia are now known to be rich in barbigerone, including M. dielsiena, M. ferruginea, M. usaramensis, and M. pachycarpa. It has also been isolated from the medicinal plant Sarcolobus globosus. Barbigerone from S. globosus is validated to have significant antioxidant property. Barbigerone exhibits profound antiplasmodial activity against the malarial parasite Plasmodium falciparum. It is also demonstrated that it has anti-cancer potential as it causes apoptosis of murine lung-cancer cells.

Medicarpin is a pterocarpan, a derivative of isoflavonoids.

Pterocarpans are derivatives of isoflavonoids found in the family Fabaceae. It is a group of compounds which can be described as benzo-pyrano-furano-benzenes which can be formed by coupling of the B ring to the 4-one position.
The applications of nanotechnology, commonly incorporate industrial, medicinal, and energy uses. These include more durable construction materials, therapeutic drug delivery, and higher density hydrogen fuel cells that are environmentally friendly. Being that nanoparticles and nanodevices are highly versatile through modification of their physiochemical properties, they have found uses in nanoscale electronics, cancer treatments, vaccines, hydrogen fuel cells, and nanographene batteries.
Sulfidogermanates or thiogermanates are chemical compounds containing anions with sulfur atoms bound to germanium. They are in the class of chalcogenidotetrelates. Related compounds include thiosilicates, thiostannates, selenidogermanates, telluridogermanates and selenidostannates.

Love Like the Galaxy is a 2022 Chinese television series. It stars Wu Lei and Zhao Lusi. The series is split into two parts, with first part starting on July 5, 2022. The second part premiered on July 27, 2022.

Callerya nitida is a species of flowering plant in the family Fabaceae, native to south-central and southeast mainland China, Hainan and Taiwan. It was first described by George Bentham in 1842 as Millettia nitida.
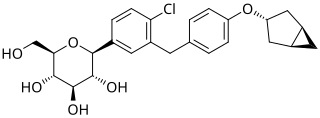
Janagliflozin is an SGLT2 inhibitor developed by Sihuan Pharmaceutical. It is approved in China for the treatment of type 2 diabetes.