 | |
| Names | |
|---|---|
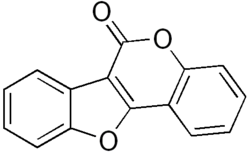
| IUPAC name Pterocarp-6a(11a)-en-6-one | |
| Systematic IUPAC name 6H-[1]Benzofuro[3,2-c][1]benzopyran-6-one | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H8O3 | |
| Molar mass | 236.22 g/mol |
| Melting point | 187 to 188 °C (369 to 370 °F; 460 to 461 K) [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Coumestan is a heterocyclic organic compound. Coumestan forms the central core of a variety of natural compounds known collectively as coumestans. Coumestans are oxidation products of pterocarpan [2] that are similar to coumarin. Coumestans, including coumestrol, a phytoestrogen, are found in a variety of plants. Food sources high in coumestans include split peas, pinto beans, lima beans, and especially alfalfa and clover sprouts. [3]
Coumestrol has about the same binding affinity for the ER-β estrogen receptor as 17β-estradiol, but much less affinity than 17α-estradiol, although the estrogenic potency of coumestrol at both receptors is much less than that of 17β-estradiol. [4]
Because of the estrogenic activity of some coumestans, a variety of syntheses have been developed that allow the preparation of coumestans so that their pharmacological effects can be explored. [5] [6]

