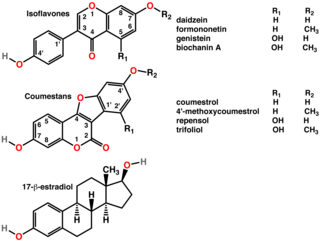
A phytoestrogen is a plant-derived xenoestrogen not generated within the endocrine system, but consumed by eating plants or manufactured foods. Also called a "dietary estrogen", it is a diverse group of naturally occurring nonsteroidal plant compounds that, because of its structural similarity to estradiol (17-β-estradiol), have the ability to cause estrogenic or antiestrogenic effects. Phytoestrogens are not essential nutrients because their absence from the diet does not cause a disease, nor are they known to participate in any normal biological function. Common foods containing phytoestrogens are soy protein, beans, oats, barley, rice, coffee, apples, carrots.
Xenoestrogens are a type of xenohormone that imitates estrogen. They can be either synthetic or natural chemical compounds. Synthetic xenoestrogens include some widely used industrial compounds, such as PCBs, BPA, and phthalates, which have estrogenic effects on a living organism even though they differ chemically from the estrogenic substances produced internally by the endocrine system of any organism. Natural xenoestrogens include phytoestrogens which are plant-derived xenoestrogens. Because the primary route of exposure to these compounds is by consumption of phytoestrogenic plants, they are sometimes called "dietary estrogens". Mycoestrogens, estrogenic substances from fungi, are another type of xenoestrogen that are also considered mycotoxins.
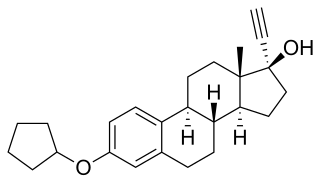
Quinestrol, also known as ethinylestradiol cyclopentyl ether (EECPE), sold under the brand name Estrovis among others, is an estrogen medication which has been used in menopausal hormone therapy, hormonal birth control, and to treat breast cancer and prostate cancer. It is taken once per week to once per month by mouth.

Mestranol, sold under the brand names Enovid, Norinyl, and Ortho-Novum among others, is an estrogen medication which has been used in birth control pills, menopausal hormone therapy, and the treatment of menstrual disorders. It is formulated in combination with a progestin and is not available alone. It is taken by mouth.

Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Estriol succinate, sold under the brand name Synapause among others, is an estrogen medication which is used in the treatment of menopausal symptoms. It is taken by mouth, in through the vagina, and by injection.

Bengt Olof Torsten Falck was a Swedish scientist, who was professor emeritus at the Faculty of Medicine at Lund University, Sweden. Falck has published numerous works in the fields of histology, endocrinology and neurobiology.

Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of the sodium salts of estrogen conjugates found in horses, such as estrone sulfate and equilin sulfate. CEEs are available in the form of both natural preparations manufactured from the urine of pregnant mares and fully synthetic replications of the natural preparations. They are formulated both alone and in combination with progestins such as medroxyprogesterone acetate. CEEs are usually taken by mouth, but can also be given by application to the skin or vagina as a cream or by injection into a blood vessel or muscle.

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

Doisynoestrol, also known as fenocycline, as well as cis-bisdehydrodoisynolic acid 7-methyl ether, is a synthetic nonsteroidal estrogen of the doisynolic acid group that is no longer marketed. It is a methyl ether of bisdehydrodoisynolic acid. Doisynoestrol was described in the literature in 1945. It has about 0.02% of the relative binding affinity of estradiol for the estrogen receptor.

Dienestrol diacetate is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It is an ester of dienestrol.

Diethylstilbestrol dipropionate (DESDP), or diethylstilbestrol dipropanoate, also known as stilboestrol dipropionate, is a synthetic nonsteroidal estrogen of the stilbestrol group that was formerly marketed widely throughout Europe. It is an ester of diethylstilbestrol with propionic acid, and is more slowly absorbed in the body than diethylstilbestrol. The medication has been said to be one of the most potent estrogens known.

Ethinylestradiol sulfonate (EES), sold under the brand names Deposiston and Turisteron among others, is an estrogen medication which has been used in birth control pills for women and in the treatment of prostate cancer in men. It has also been investigated in the treatment of breast cancer in women. The medication was combined with norethisterone acetate in birth control pills. EES is taken by mouth once per week.

17α-Methylprogesterone (17α-MP), or 17α-methylpregn-4-ene-3,20-dione, is a steroidal progestin related to progesterone that was synthesized and characterized in 1949 but was never marketed. Along with ethisterone (1938) and 19-norprogesterone (1951), 17α-MP was one of the earliest derivatives of progesterone to be identified as possessing progestogenic activity. Similarly to progesterone and derivatives like 17α-hydroxyprogesterone and 19-norprogesterone, 17α-MP was found to possess poor oral bioavailability, but showed improved progestogenic activity relative to progesterone when administered via other routes. In addition to its activity as a progestogen, 17α-MP has also been found to possess some antiglucocorticoid activity.

Estradiol sulfamate, or estradiol-3-O-sulfamate, is a steroid sulfatase (STS) inhibitor which is under development for the treatment of endometriosis. It is the C3 sulfamate ester of estradiol, and was originally thought to be a prodrug of estradiol.
Gestadienol acetate an orally active progestin which was described in the literature in 1967 and was never marketed. It has no androgenic or estrogenic effects. The effects of gestadienol acetate on the endometrium and its general pharmacology were studied in a clinical trial in women. It has also been studied in a clinical trial for benign prostatic hyperplasia in men, but was ineffective.

Estriol (E3), sold under the brand name Ovestin among others, is an estrogen medication and naturally occurring steroid hormone which is used in menopausal hormone therapy. It is also used in veterinary medicine as Incurin to treat urinary incontinence due to estrogen deficiency in dogs. The medication is taken by mouth in the form of tablets, as a cream that is applied to the skin, as a cream or pessary that is applied in the vagina, and by injection into muscle.

Estradiol 17β-tetrahydropyranyl ether is a synthetic estrogen and estrogen ether which was never marketed. It has been reported to possess improved oral activity relative to estradiol. One study in animals found that it had 15 times the oral activity of estradiol.

Estradiol 3-tetrahydropyranyl ether is a synthetic estrogen and estrogen ether which was never marketed. It has been reported to possess improved oral activity relative to estradiol. One study in animals found that it had 15 times the oral activity of estradiol.
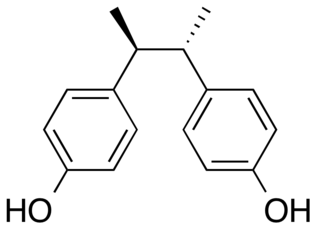
Butestrol, or racemic butestrol (rac-butestrol) is a synthetic nonsteroidal estrogen which was never marketed. It is structurally related to diethylstilbestrol and other stilbestrols.



















