
Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

Metandienone, also known as methandienone or methandrostenolone and sold under the brand name Dianabol (D-Bol) among others, is an androgen and anabolic steroid (AAS) medication which is still quite often used because of its affordability and effectiveness for bulking cycles. It is also used non-medically for physique- and performance-enhancing purposes. It is often taken by mouth.

Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis. The medication is available alone and is not formulated or used in combination with other medications. It is taken by mouth.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.
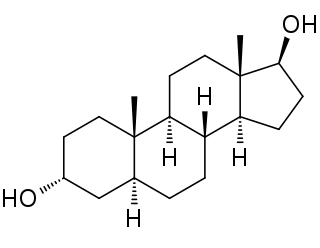
3α-Androstanediol also known as 5α-androstane-3α,17β-diol and sometimes shortened in the literature to 3α-diol, is an endogenous steroid hormone and neurosteroid and a metabolite of androgens like dihydrotestosterone (DHT).

Estetrol (E4), or oestetrol, is one of the four natural estrogenic steroid hormones found in humans, along with estrone (E1), estradiol (E2), and estriol (E3), estetrol is a major estrogen in the body. In contrast to estrone and estradiol, estetrol is a native estrogen of fetal life. Estetrol is produced exclusively by the fetal liver and is found in detectable levels only during pregnancy, with relatively high levels in the fetus and lower levels in the maternal circulation.

Noretynodrel, or norethynodrel, sold under the brand name Enovid among others, is a progestin medication which was previously used in birth control pills and in the treatment of gynecological disorders but is now no longer marketed. It was available both alone and in combination with an estrogen. The medication is taken by mouth.

3β-Androstanediol, also known as 5α-androstane-3β,17β-diol, and sometimes shortened in the literature to 3β-diol, is an endogenous steroid hormone and a metabolite of androgens like dehydroepiandrosterone (DHEA) and dihydrotestosterone (DHT).

11α-Hydroxyprogesterone (11α-OHP), or 11α-hydroxypregn-4-ene-3,20-dione is an endogenous steroid and metabolite of progesterone. It is a weak antiandrogen, and is devoid of androgenic, estrogenic, and progestogenic activity.

8,9-Dehydroestrone, or Δ8-estrone, also known as estra-1,3,5(10),8-tetraen-3-ol-17-one, is a naturally occurring estrogen found in horses which is closely related to equilin, equilenin, and estrone, and, as the 3-sulfate ester sodium salt, is a minor constituent (3.5%) of conjugated estrogens (Premarin). It produces 8,9-dehydro-17β-estradiol as an important active metabolite, analogously to conversion of estrone or estrone sulfate into estradiol. The compound was first described in 1997. In addition to 8,9-dehydroestrone and 8,9-dehydro-17β-estradiol, 8,9-dehydro-17α-estradiol is likely also to be present in conjugated estrogens, but has not been identified at this time.

Δ4-Tibolone, also known as 7α-methylnorethisterone or as 7α-methyl-17α-ethynyl-19-nortestosterone, is a synthetic androgen and progestin which was never marketed. The compound is a major active metabolite of tibolone, which itself is a prodrug of δ4-tibolone along with 3α-hydroxytibolone and 3β-hydroxytibolone. Tibolone and δ4-tibolone are thought to be responsible for the androgenic and progestogenic activity of tibolone, while 3α-hydroxytibolone and 3β-hydroxytibolone are thought to be responsible for its estrogenic activity.

7β-Hydroxyepiandrosterone (7β-OH-EPIA), also known as 5α-androstan-3β,7β-diol-17-one, is an endogenous androgen, estrogen, and neurosteroid that is produced from dehydroepiandrosterone and epiandrosterone. It has neuroprotective effects and, along with 7α-hydroxyepiandrosterone, may mediate the neuroprotective effects of DHEA. 7β-OH-EPIA may act as a highly potent antagonist of the G protein-coupled estrogen receptor (GPER).
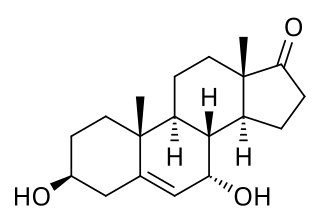
7α-Hydroxydehydroepiandrosterone, also known as 3β,7α-dihydroxyandrost-5-ene-17-one, is an endogenous, naturally occurring steroid and a major metabolite of dehydroepiandrosterone (DHEA) that is formed by CYP7B1 in tissues such as the prostate gland and by CYP3A4 in the liver. The major metabolic pathway of DHEA outside the liver is via 7-hydroxylation into 7α-OH-DHEA and 7β-OH-DHEA. 7α-OH-DHEA has weak estrogenic activity, selectively activating the estrogen receptor ERβ. In addition, 7α-OH-DHEA may be responsible for the known antiglucocorticoid effects of DHEA.

7β-Hydroxydehydroepiandrosterone, also known as 3β,7β-dihydroxyandrost-5-ene-17-one, is an endogenous, naturally occurring steroid and a metabolite of dehydroepiandrosterone (DHEA). The major metabolic pathway of DHEA outside the liver is via 7-hydroxylation into 7α-OH-DHEA and 7β-OH-DHEA. 7β-OH-DHEA has weak antiestrogenic activity, selectively antagonizing the estrogen receptor ERβ.

17α-Ethynyl-3α-androstanediol, also known as 17α-ethynyl-5α-androstane-3α,17β-diol, is a synthetic androstane steroid and a 17α-substituted derivative of 3α-androstanediol which was never marketed. It was under development for the treatment of prostate cancer but was discontinued.

5α-Dihydronorethisterone is a major active metabolite of norethisterone (norethindrone). Norethisterone is a progestin with additional weak androgenic and estrogenic activity. 5α-DHNET is formed from norethisterone by 5α-reductase in the liver and other tissues.

3α-Hydroxytibolone is a synthetic steroidal estrogen which was never marketed. Along with 3β-hydroxytibolone and δ4-tibolone, it is a major active metabolite of tibolone, and 3α-hydroxytibolone and 3β-hydroxytibolone are thought to be responsible for the estrogenic activity of tibolone.

5α-Dihydrolevonorgestrel (5α-DHLNG) is an active metabolite of the progestin levonorgestrel which is formed by 5α-reductase. It has about one-third of the affinity of levonorgestrel for the progesterone receptor. In contrast to levonorgestrel, the compound has both progestogenic and antiprogestogenic activity, and hence has a selective progesterone receptor modulator-like profile of activity. This is analogous to the case of norethisterone and 5α-dihydronorethisterone. In addition to the progesterone receptor, 5α-DHLNG interacts with the androgen receptor. It has similar affinity for the androgen receptor relative to levonorgestrel, and has androgenic effects similarly to levonorgestrel and testosterone. 5α-DHLNG is further transformed into 3α,5α- and 3β,5α-THLNG, which bind weakly to the estrogen receptor and have weak estrogenic activity. These metabolites are considered to be responsible for the weak estrogenic activity of high doses of levonorgestrel.

The structure–activity relationships (SAR) of anabolic steroids (AAS) have been extensively studied.

5α-Dihydroethisterone is an active metabolite of the formerly clinically used but now-discontinued progestin ethisterone and the experimental and never-marketed hormonal antineoplastic agent ethynylandrostanediol (HE-3235). Its formation from its parent drugs is catalyzed by 5α-reductase in tissues that express the enzyme in high amounts like the liver, skin, hair follicles, and prostate gland. 5α-DHET has significant affinity for steroid hormone receptors and may contribute importantly to the activities of its parent drugs.




















