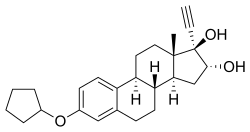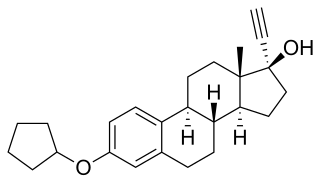
Quinestrol, also known as ethinylestradiol cyclopentyl ether (EECPE), sold under the brand name Estrovis among others, is an estrogen medication which has been used in menopausal hormone therapy, hormonal birth control, and to treat breast cancer and prostate cancer. It is taken once per week to once per month by mouth.

Quinbolone, sold under the brand names Anabolicum and Anabolvis, is an androgen and anabolic steroid (AAS) which was previously marketed in Italy. It was developed by Parke-Davis as a viable orally administered AAS with little or no liver toxicity.
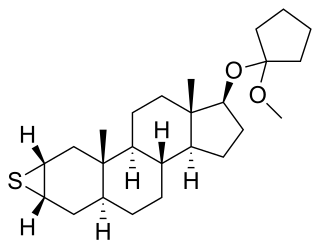
Mepitiostane, sold under the brand name Thioderon, is an orally active antiestrogen and anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which is marketed in Japan as an antineoplastic agent for the treatment of breast cancer. It is a prodrug of epitiostanol. The drug was patented and described in 1968.
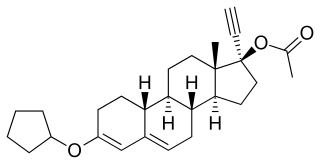
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

Penmesterol, or penmestrol, also known as 17α-methyltestosterone 3-cyclopentyl enol ether, is a synthetic, orally active anabolic-androgenic steroid (AAS) that was developed in the early 1960s. It is the 3-cyclopentyl enol ether of methyltestosterone.

Quinestradol, also known as quinestradiol or quinestriol, as well as estriol 3-cyclopentyl ether (E3CPE), is a synthetic estrogen and estrogen ether which is no longer marketed. It is the 3-cyclopentyl ether of estriol. The medication has been studied in the treatment of stress incontinence in elderly women, with effectiveness observed.

Orestrate, also known as estradiol 3-propionate 17β-(1-cyclohexenyl) ether, is an estrogen medication and estrogen ester which was never marketed. It is the C3 propionate ester and C17β-(1-cyclohexenyl) ether of estradiol.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.

Doisynoestrol, also known as fenocycline, as well as cis-bisdehydrodoisynolic acid 7-methyl ether, is a synthetic nonsteroidal estrogen of the doisynolic acid group that is no longer marketed. It is a methyl ether of bisdehydrodoisynolic acid. Doisynoestrol was described in the literature in 1945. It has about 0.02% of the relative binding affinity of estradiol for the estrogen receptor.

Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth.

Pentagestrone, also known as 17α-hydroxyprogesterone 3-cyclopentyl enol ether, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. An acetate ester, pentagestrone acetate, has been marketed for clinical use. Pentagestrone was described in the literature in 1960.

Pentagestrone acetate (PGA), sold under the brand names Gestovis and Gestovister, is a progestin which was described in the literature in 1960 and was introduced by Vister in Italy in 1961. It is the 3-cyclopentyl enol ether of 17α-hydroxyprogesterone acetate. PGA, along with quingestrone, is said to have very similar properties to those of dydrogesterone, a pure progestogen and close analogue of progesterone.

Ethinylestriol (EE3), or 17α-ethynylestriol, also known as 17α-ethynylestra-1,3,5(10)-triene-3,16α,17β-triol, is a synthetic estrogen which was never marketed. Nilestriol, the 3-cyclopentyl ether of ethinylestriol, is a prodrug of ethinylestriol, and is a more potent estrogen in comparison, but, in contrast to ethinylestriol, has been marketed. Ethinylestriol has been found to reduce the risk of 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary cancer when given as a prophylactic in animal models, while other estrogens like ethinylestradiol and diethylstilbestrol were ineffective.

Mestilbol, also known as diethylstilbestrol monomethyl ether, is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It was developed by Wallace & Tiernan Company, patented in 1940, and introduced for medical use in the 1940s, but is now no longer marketed. Mestilbol was available both as oral tablets and in oil for intramuscular injection. The drug is gradually demethylated in the body into diethylstilbestrol and hence is a prodrug of diethylstilbestrol. Mestilbol is a highly active estrogen, although somewhat less so than diethylstilbestrol, but is longer-lasting in comparison.
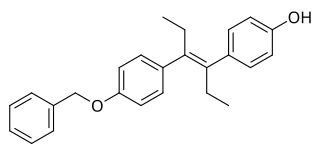
Diethylstilbestrol monobenzyl ether, also known as benzelstilbestrol, is a synthetic, nonsteroidal estrogen of the stilbestrol group and an ether of diethylstilbestrol (DES) that is described as a pituitary gland inhibitor (antigonadotropin) and was formerly marketed but is now no longer available. It was first synthesized by Wallace & Tiernan Company in 1952, and was described by them as having only weak estrogenic activity. The drug was used to treat gynecological conditions and infertility in women.

Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed. A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.

Methyltestosterone 3-hexyl ether, or 17α-methyltestosterone 3-hexyl enol ether, also known as 17α-methylandrost-3,5-dien-17β-ol-3-one 3-hexyl ether, is a synthetic anabolic-androgenic steroid and an androgen ether – specifically, the 3-hexyl ether of methyltestosterone.

Estrone methyl ether, or estrone 3-methyl ether, is a synthetic estrogen and estrogen ether – specifically, the C3 methyl ether of estrone – which was never marketed. It has been used to synthesize mestranol.