Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones occur in many plant species, but are especially high in soybeans.

Stigmasterol – a plant sterol (phytosterol) – is among the most abundant of plant sterols, having a major function to maintain the structure and physiology of cell membranes. In the European Union, it is a food additive listed with E number E499, and may be used in food manufacturing to increase the phytosterol content, potentially lowering the levels of LDL cholesterol.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility.
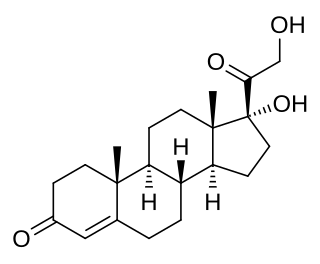
11-Deoxycortisol, also known as cortodoxone (INN), cortexolone as well as 17α,21-dihydroxyprogesterone or 17α,21-dihydroxypregn-4-ene-3,20-dione, is an endogenous glucocorticoid steroid hormone, and a metabolic intermediate toward cortisol. It was first described by Tadeusz Reichstein in 1938 as Substance S, thus has also been referred to as Reichstein's Substance S or Compound S.
In enzymology, a 3,9-dihydroxypterocarpan 6a-monooxygenase (EC 1.14.13.28) is an enzyme that catalyzes the chemical reaction
In enzymology, glyceollin synthase is an enzyme that catalyzes the last committed step in glyceollin biosynthesis. This enzyme has been classified as a cytochrome dependent monooxygenase. It uses cyclization of prenyl residue to convert glyceollidins into glyceollins.
In enzymology, a naringenin 8-dimethylallyltransferase is an enzyme that catalyzes the chemical reaction

Dual specificity mitogen-activated protein kinase kinase 5 is an enzyme that in humans is encoded by the MAP2K5 gene.

Prenylated flavonoids or prenylflavonoids are a sub-class of flavonoids. They are widely distributed throughout the plant kingdom. Some are known to have phytoestrogenic or antioxidant properties. They are given in the list of adaptogens in herbalism. Chemically they have a prenyl group attached to their flavonoid backbone. It is usually assumed that the addition of hydrophobic prenyl groups facilitate attachment to cell membranes. Prenylation may increase the potential activity of its original flavonoid.

Xanthohumol is a natural product found in the female inflorescences of Humulus lupulus, also known as hops. This compound is also found in beer and belongs to a class of compounds that contribute to the bitterness and flavor of hops. Xanthohumol is a prenylated chalconoid, biosynthesized by a type III polyketide synthase (PKS) and subsequent modifying enzymes.

Pterocarpans are derivatives of isoflavonoids found in the family Fabaceae. It is a group of compounds which can be described as benzo-pyrano-furano-benzenes which can be formed by coupling of the B ring to the 4-one position.
Glyceollins are a family of prenylated pterocarpans found in ineffective types of nodule in soybean in response to symbiotic infection.

Glycinol is a pterocarpan, a type of natural phenol. It is a phytoalexin found in the soybean. It is formed by the cyclisation of daidzein.

Glyceollin III is a glyceollin, a type of pterocarpan, found in the soybean. It has an antiestrogenic effect. In soil, it has an antifungal activity against Aspergillus sojae.

Aspergillus sojae is a species of fungus in the genus Aspergillus.
Trihydroxypterocarpan dimethylallyltransferase is an enzyme with systematic name dimethylallyl-diphosphate:(6aS,11aS)-3,6a,9-trihydroxypterocarpan dimethylallyltransferase. This enzyme catalyses the following chemical reaction

Pisatin (3-hydroxy-7-methoxy-4′,5′-methylenedioxy-chromanocoumarane) is the major phytoalexin made by the pea plant Pisum sativum. It was the first phytoalexin to be purified and chemically identified. The molecular formula is C17H14O6.

2-Hydroxyestrone (2-OHE1), also known as estra-1,3,5(10)-trien-2,3-diol-17-one, is an endogenous, naturally occurring catechol estrogen and a major metabolite of estrone and estradiol. It is formed irreversibly from estrone in the liver and to a lesser extent in other tissues via 2-hydroxylation mediated by cytochrome P450 enzymes, mainly the CYP3A and CYP1A subfamilies. 2-OHE1 is the most abundant catechol estrogen in the body.
Martin Parniske is a German biologist with a specialisation in genetics, microbiology and biochemistry. He is university professor and head of the Institute of Genetics at the Faculty of Biology of the Ludwig Maximilian University of Munich. Parniske's scientific focus is on the molecular interaction between plants and symbiotic and pathogenic organisms including bacteria, fungi, oomycetes and insects.
ZB716, also known as fulvestrant-3-boronic acid, is a synthetic, steroidal, orally active antiestrogen which is under development for the treatment of estrogen receptor (ER)-positive metastatic breast cancer. The drug is a silent antagonist of the ERα (IC50 = 4.1 nM) as well as a selective estrogen receptor degrader (SERD). It is an analogue and prodrug of fulvestrant in which the C3 hydroxyl group has been replaced with a boronic acid moiety. In accordance, the two drugs have similar pharmacodynamic properties. However, whereas fulvestrant is not orally active and must be administered via intramuscular injection, ZB716 is less susceptible to first-pass metabolism, and in relation to this, is orally active.












