
Toremifene, sold under the brand name Fareston among others, is a medication which is used in the treatment of advanced breast cancer in postmenopausal women. It is taken by mouth.

Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5–6 mg/kg.
Antiestrogens, also known as estrogen antagonists or estrogen blockers, are a class of drugs which prevent estrogens like estradiol from mediating their biological effects in the body. They act by blocking the estrogen receptor (ER) and/or inhibiting or suppressing estrogen production. Antiestrogens are one of three types of sex hormone antagonists, the others being antiandrogens and antiprogestogens. Antiestrogens are commonly used to stop steroid hormones, estrogen, from binding to the estrogen receptors leading to the decrease of estrogen levels. Decreased levels of estrogen can lead to complications in sexual development.

Nafoxidine or nafoxidine hydrochloride is a nonsteroidal selective estrogen receptor modulator (SERM) or partial antiestrogen of the triphenylethylene group that was developed for the treatment of advanced breast cancer by Upjohn in the 1970s but was never marketed. It was developed at around the same time as tamoxifen and clomifene, which are also triphenylethylene derivatives. The drug was originally synthesized by the fertility control program at Upjohn as a postcoital contraceptive, but was subsequently repurposed for the treatment of breast cancer. Nafoxidine was assessed in clinical trials in the treatment of breast cancer and was found to be effective. However, it produced side effects including ichthyosis, partial hair loss, and phototoxicity of the skin in almost all patients, and this resulted in the discontinuation of its development.

Trioxifene, or as the salt trioxifene mesylate (USAN), is a selective estrogen receptor modulator (SERM) with competitive binding activity against estradiol for the ERα and antagonistic activity against ERα-mediated gene expression, that was under preclinical and clinical development by Eli Lilly and Company for breast cancer and prostate cancer, but was abandoned. Its affinity for the rat estrogen receptor was reported to be 20% relative to estradiol.

Anol, also known as p-hydroxypropenylbenzene, is a simple phenol that was derived via demethylation from anethole, an estrogenic constituent of anise and fennel, by Sir Charles Dodds in 1937. It was reported to possess extremely potent estrogenic activity on par with that of steroidal estrogens like estrone, with a dose of 1 μg inducing estrus in rats. However, subsequent studies with different preparations of anol failed to confirm these findings, and it was found that dimerization of anol into dianol and hexestrol can rapidly occur and that the latter impurity was responsible for the highly potent estrogenic effects. Dodds later synthesized the structurally related and extremely potent estrogen diethylstilbestrol in 1938.

A nonsteroidal estrogen is an estrogen with a nonsteroidal chemical structure. The most well-known example is the stilbestrol estrogen diethylstilbestrol (DES). Although nonsteroidal estrogens formerly had an important place in medicine, they have gradually fallen out of favor following the discovery of toxicities associated with high-dose DES starting in the early 1970s, and are now almost never used. On the other hand, virtually all selective estrogen receptor modulators (SERMs) are nonsteroidal, with triphenylethylenes like tamoxifen and clomifene having been derived from DES, and these drugs remain widely used in medicine for the treatment of breast cancer among other indications. In addition to pharmaceutical drugs, many xenoestrogens, including phytoestrogens, mycoestrogens, and synthetic endocrine disruptors like bisphenol A, are nonsteroidal substances with estrogenic activity.

Hexestrol, sold under the brand name Synestrol among others, is a nonsteroidal estrogen which was previously used for estrogen replacement therapy and in the treatment of certain hormone-dependent cancers as well as gynecological disorders but is mostly no longer marketed. It has also been used in the form of esters such as hexestrol diacetate and hexestrol dipropionate. Hexestrol and its esters are taken by mouth, held under the tongue, or via injection into muscle.

Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity. Its estrogenic effects were discovered in 1937. TPE was derived from structural modification of the more potent estrogen diethylstilbestrol, which is a member of the stilbestrol group of nonsteroidal estrogens.

Estrobin, also known as α,α-di(p-ethoxyphenyl)-β-phenylbromoethylene and commonly abbreviated as DBE, is a synthetic, nonsteroidal estrogen of the triphenylethylene group that was never marketed. Chlorotrianisene, and subsequently clomifene and tamoxifen, were derived from it. Estrobin, similarly to other triphenylethylenes, is very lipophilic and hence very long-lasting in its duration of action. Similarly to chlorotrianisene, estrobin behaves a prodrug to a much more potent estrogen in the body.

Ethamoxytriphetol is a synthetic nonsteroidal antiestrogen that was studied clinically in the late 1950s and early 1960s but was never marketed. MER-25 was first reported in 1958, and was the first antiestrogen to be discovered. It has been described as "essentially devoid of estrogenic activity" and as having "very low estrogenic activity in all species tested". However, some estrogenic effects in the uterus have been observed, so it is not a pure antiestrogen but is, instead, technically a selective estrogen receptor modulator (SERM). For all intents and purposes, it is a nearly pure antiestrogen, however.

Dianethole is a naturally occurring organic compound that is found in anise and fennel. It is a dimeric polymer of anethole. It has estrogenic activity, and along with anethole and photoanethole, may be responsible for the estrogenic effects of anise and fennel. These compounds bear resemblance to the estrogens stilbene and diethylstilbestrol, which may explain their estrogenic activity. In fact, it is said that diethylstilbestrol and related drugs were originally modeled after dianethole and photoanethole.

Dianol is a synthetic, nonsteroidal estrogen that was never marketed. It is a dimer and impurity of anol, and was, along with hexestrol, involved in erroneous findings of highly potent estrogenic activity with anol. Although a potent estrogen, it requires a dose of 100 μg to show activity, whereas hexestrol shows activity with a mere dose of 0.2 μg.
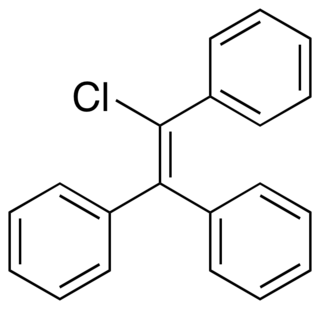
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.
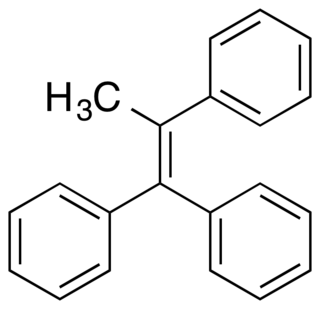
Triphenylmethylethylene, also known as methyltriphenylethylene or as triphenylpropene, is a synthetic nonsteroidal estrogen of the triphenylethylene group that is related to triphenylchloroethylene and was never marketed. Along with diethylstilbestrol and triphenylchoroethylene, triphenylmethylethylene was studied in 1944 by Sir Alexander Haddow for the treatment of breast cancer, and this is historically notable in that it was the first time that high-dose estrogens were found to be effective in the treatment of breast cancer. However, while diethylstilbestrol and triphenylchloroethylene were found to be significantly effective, triphenylmethylethylene was less effective and showed a favorable response in only 1 of 4 treated cases.

Droloxifene, also known as 3-hydroxytamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed originally in Germany and later in Japan for the treatment of breast cancer, osteoporosis in men and postmenopausal women, and cardiovascular disorders but was abandoned and never marketed. It reached phase II and phase III clinical trials for these indications before development was discontinued in 2000. The drug was found to be significantly less effective than tamoxifen in the treatment of breast cancer in two phase III clinical trials.

Photoanethole is a naturally occurring organic compound that is found in anise and fennel. It has estrogenic activity, and along with anethole and dianethole, may be responsible for the estrogenic effects of anise and fennel. These compounds bear resemblance to the estrogens stilbene and diethylstilbestrol, which may explain their estrogenic activity. In fact, it is said that diethylstilbestrol and related drugs were originally modeled after photoanethole and dianethole.
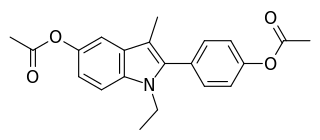
Zindoxifene is a nonsteroidal selective estrogen receptor modulator (SERM) that was under development in the 1980s and early 1990s for the treatment of breast cancer but was not marketed. It showed estrogenic-like activity in preclinical studies and failed to demonstrate effectiveness as a treatment for breast cancer in clinical trials. Zindoxifene was the lead compound of the distinct 2-phenylindole class of SERMs, and the marketed SERM bazedoxifene was derived from the major active metabolite of zindoxifene, D-15414. Zindoxifene was first described in 1984.
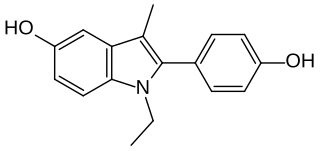
D-15414 is a nonsteroidal weak estrogen of the 2-phenylindole group which was never marketed. It is the major metabolite of the selective estrogen receptor modulator (SERM) zindoxifene (D-16726). D-15414 has high affinity for the estrogen receptor (ER) and inhibits the growth of ER-positive MCF-7 breast cancer cells in vitro. However, contradictorily, subsequent research found that the drug produced fully estrogenic effects in vitro similarly to but less actively than estradiol, with no antiestrogenic activity observed. The reason for the discrepancy between the findings is unclear, though may be due to methodology. The unexpected estrogenic activity of D-15414 may be responsible for the failure of zindoxifene in clinical trials as a treatment for breast cancer.

Nitromifene (INNTooltip International Nonproprietary Name; also as the citrate salt nitromifene citrate (USANTooltip United States Adopted Name), developmental code names CI-628, CN-5518, CN-55945) is a nonsteroidal selective estrogen receptor modulator (SERM) related to triphenylethylenes like tamoxifen that was never marketed. It is a mixture of (E)- and (Z)-isomers that possess similar antiestrogenic activity. The drug was described in 1966. Along with tamoxifen, nafoxidine, and clomifene, it was one of the earliest SERMs.



















