
Clomifene, also known as clomiphene, is a medication used to treat infertility in women who do not ovulate, including those with polycystic ovary syndrome. It is taken by mouth.

Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonists/antagonists (ERAAs), are a class of drugs that act on estrogen receptors (ERs). Compared to pure ER agonists–antagonists, SERMs are more tissue-specific, allowing them to selectively inhibit or stimulate estrogen-like action in various tissues.

Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and men. It is also being studied for other types of cancer. It has been used for Albright syndrome. Tamoxifen is typically taken daily by mouth for five years for breast cancer.

Estrogen receptors (ERs) are proteins found in cells that function as receptors for the hormone estrogen (17β-estradiol). There are two main classes of ERs. The first includes the intracellular estrogen receptors, namely ERα and ERβ, which belong to the nuclear receptor family. The second class consists of membrane estrogen receptors (mERs), such as GPER (GPR30), ER-X, and Gq-mER, which are primarily G protein-coupled receptors. This article focuses on the nuclear estrogen receptors.

Toremifene, sold under the brand name Fareston among others, is a medication which is used in the treatment of advanced breast cancer in postmenopausal women. It is taken by mouth.

Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis. The medication is available alone and is not formulated or used in combination with other medications. It is taken by mouth.

Virgil Craig Jordan,, was an American and British scientist specializing in drugs for breast cancer treatment and prevention. He was Professor of Breast Medical Oncology, and Professor of Molecular and Cellular Oncology at the University of Texas MD Anderson Cancer Center, Houston, Texas. Previously, he was Scientific Director and Vice Chairman of Oncology at the Lombardi Comprehensive Cancer Center of Georgetown University. Jordan was the first to discover the breast cancer prevention properties of tamoxifen and the scientific principles for adjuvant therapy with antihormones. His later work branched out into the prevention of multiple diseases in women with the discovery of the drug group, selective estrogen receptor modulator (SERMs). He later worked on developing a new Hormone Replacement Therapy (HRT) for post-menopausal women that prevents breast cancer and does not increase the risk of breast cancer.
Antiestrogens, also known as estrogen antagonists or estrogen blockers, are a class of drugs which prevent estrogens like estradiol from mediating their biological effects in the body. They act by blocking the estrogen receptor (ER) and/or inhibiting or suppressing estrogen production. Antiestrogens are one of three types of sex hormone antagonists, the others being antiandrogens and antiprogestogens. Antiestrogens are commonly used to stop steroid hormones, estrogen, from binding to the estrogen receptors leading to the decrease of estrogen levels. Decreased levels of estrogen can lead to complications in sexual development.
The estrogen receptor test (ERT) is a laboratory test to determine whether cancer cells have estrogen receptors. This information can guide treatment of the cancer.

Afimoxifene, also known as 4-hydroxytamoxifen (4-OHT) and by its tentative brand name TamoGel, is a selective estrogen receptor modulator (SERM) of the triphenylethylene group and an active metabolite of tamoxifen. The drug is under development under the tentative brand name TamoGel as a topical gel for the treatment of hyperplasia of the breast. It has completed a phase II clinical trial for cyclical mastalgia, but further studies are required before afimoxifene can be approved for this indication and marketed.

Nafoxidine or nafoxidine hydrochloride is a nonsteroidal selective estrogen receptor modulator (SERM) or partial antiestrogen of the triphenylethylene group that was developed for the treatment of advanced breast cancer by Upjohn in the 1970s but was never marketed. It was developed at around the same time as tamoxifen and clomifene, which are also triphenylethylene derivatives. The drug was originally synthesized by the fertility control program at Upjohn as a postcoital contraceptive, but was subsequently repurposed for the treatment of breast cancer. Nafoxidine was assessed in clinical trials in the treatment of breast cancer and was found to be effective. However, it produced side effects including ichthyosis, partial hair loss, and phototoxicity of the skin in almost all patients, and this resulted in the discontinuation of its development.

Ethamoxytriphetol is a synthetic nonsteroidal antiestrogen that was studied clinically in the late 1950s and early 1960s but was never marketed. MER-25 was first reported in 1958, and was the first antiestrogen to be discovered. It has been described as "essentially devoid of estrogenic activity" and as having "very low estrogenic activity in all species tested". However, some estrogenic effects in the uterus have been observed, so it is not a pure antiestrogen but is, instead, technically a selective estrogen receptor modulator (SERM). For all intents and purposes, it is a nearly pure antiestrogen, however.

Brilanestrant (INN) is a nonsteroidal combined selective estrogen receptor modulator (SERM) and selective estrogen receptor degrader (SERD) that was discovered by Aragon Pharmaceuticals and was under development by Genentech for the treatment of locally advanced or metastatic estrogen receptor (ER)-positive breast cancer.

Etacstil is an orally active, nonsteroidal, combined selective estrogen receptor modulator (SERM) and selective estrogen receptor degrader (SERD) that was developed for the treatment of estrogen receptor-positive breast cancer. It was shown to overcome antiestrogen resistance in breast cancer by altering the shape of the estrogen receptor, thus exhibiting SERD properties. Etacstil is a tamoxifen derivative and one of the first drugs to overcome tamoxifen-resistance. It is the predecessor of GW-7604, of which etacstil is a prodrug. This is analogous to the case of tamoxifen being a prodrug of afimoxifene (4-hydroxytamoxifen).

Endoxifen, also known as 4-hydroxy-N-desmethyltamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group as well as a protein kinase C (PKC) inhibitor. It is under development for the treatment of estrogen receptor-positive breast cancer and for the treatment of mania in bipolar disorder. It is taken by mouth.
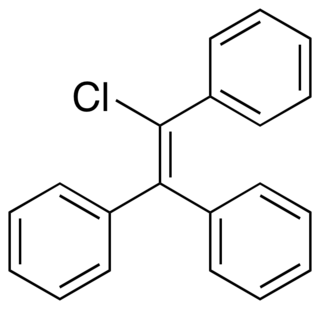
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.

Droloxifene, also known as 3-hydroxytamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed originally in Germany and later in Japan for the treatment of breast cancer, osteoporosis in men and postmenopausal women, and cardiovascular disorders but was abandoned and never marketed. It reached phase II and phase III clinical trials for these indications before development was discontinued in 2000. The drug was found to be significantly less effective than tamoxifen in the treatment of breast cancer in two phase III clinical trials.
Tesmilifene, also known as N,N-diethyl-2-(4-phenylmethyl)ethanamine (DPPE), is a small-molecule antineoplastic drug and chemopotentiator that was under development by YM BioSciences for the treatment of breast cancer in the 2000s but was never marketed. It reached phase III clinical trials for advanced/metastatic breast cancer before development was discontinued.
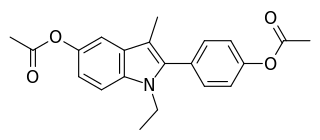
Zindoxifene is a nonsteroidal selective estrogen receptor modulator (SERM) that was under development in the 1980s and early 1990s for the treatment of breast cancer but was not marketed. It showed estrogenic-like activity in preclinical studies and failed to demonstrate effectiveness as a treatment for breast cancer in clinical trials. Zindoxifene was the lead compound of the distinct 2-phenylindole class of SERMs, and the marketed SERM bazedoxifene was derived from the major active metabolite of zindoxifene, D-15414. Zindoxifene was first described in 1984.















