
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues. However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors, and acting as a neurosteroid and modulator of neurotrophic factor receptors.

An androgen is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This includes the embryological development of the primary male sex organs, and the development of male secondary sex characteristics at puberty. Androgens are synthesized in the testes, the ovaries, and the adrenal glands.
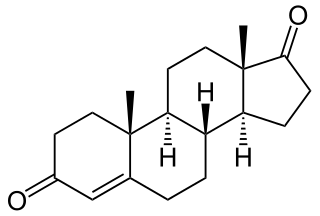
Androstenedione, or 4-androstenedione, also known as androst-4-ene-3,17-dione, is an endogenous weak androgen steroid hormone and intermediate in the biosynthesis of estrone and of testosterone from dehydroepiandrosterone (DHEA). It is closely related to androstenediol (androst-5-ene-3β,17β-diol).
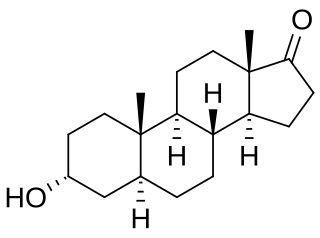
Androsterone, or 3α-hydroxy-5α-androstan-17-one, is an endogenous steroid hormone, neurosteroid, and putative pheromone. It is a weak androgen with a potency that is approximately 1/7 that of testosterone. Androsterone is a metabolite of testosterone and dihydrotestosterone (DHT). In addition, it can be converted back into DHT via 3α-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase, bypassing conventional intermediates such as androstanedione and testosterone, and as such, can be considered to be a metabolic intermediate in its own right.

Estriol (E3), also spelled oestriol, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estrone. Levels of estriol in women who are not pregnant are almost undetectable. However, during pregnancy, estriol is synthesized in very high quantities by the placenta and is the most produced estrogen in the body by far, although circulating levels of estriol are similar to those of other estrogens due to a relatively high rate of metabolism and excretion. Relative to estradiol, both estriol and estrone have far weaker activity as estrogens.

Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effects are mediated by slow genomic mechanisms through nuclear receptors as well as by fast nongenomic mechanisms through membrane-associated receptors and signaling cascades. The polypeptide hormones luteinizing hormone, follicle-stimulating hormone and gonadotropin-releasing hormone – each associated with the gonadotropin axis – are usually not regarded as sex hormones, although they play major sex-related roles.
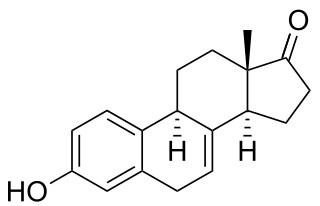
Equilin is a naturally occurring estrogen sex hormone found in horses as well as a medication. It is one of the estrogens present in the estrogen combination drug preparations known as conjugated estrogens and esterified estrogens. CEEs is the most commonly used form of estrogen medications in hormone replacement therapy (HRT) for menopausal symptoms in the United States. Estrone sulfate is the major estrogen in CEEs while equilin sulfate is the second major estrogen in the formulation, present as about 25% of the total.
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.

Dehydroepiandrosterone sulfate, abbreviated as DHEA sulfate or DHEA-S, also known as androstenolone sulfate, is an endogenous androstane steroid that is produced by the adrenal cortex. It is the 3β-sulfate ester and a metabolite of dehydroepiandrosterone (DHEA) and circulates in far greater relative concentrations than DHEA. The steroid is hormonally inert and is instead an important neurosteroid and neurotrophin.
In enzymology, a steroid sulfotransferase is an enzyme that catalyzes the chemical reaction

Estrone sulfate, also known as E1S, E1SO4 and estrone 3-sulfate, is a natural, endogenous steroid and an estrogen ester and conjugate.

Pregnenolone sulfate is an endogenous excitatory neurosteroid that is synthesized from pregnenolone. It is known to have cognitive and memory-enhancing, antidepressant, anxiogenic, and proconvulsant effects.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.

Estradiol sulfate (E2S), or 17β-estradiol 3-sulfate, is a natural, endogenous steroid and an estrogen ester. E2S itself is biologically inactive, but it can be converted by steroid sulfatase into estradiol, which is a potent estrogen. Simultaneously, estrogen sulfotransferases convert estradiol to E2S, resulting in an equilibrium between the two steroids in various tissues. Estrone and E2S are the two immediate metabolic sources of estradiol. E2S can also be metabolized into estrone sulfate (E1S), which in turn can be converted into estrone and estradiol. Circulating concentrations of E2S are much lower than those of E1S. High concentrations of E2S are present in breast tissue, and E2S has been implicated in the biology of breast cancer via serving as an active reservoir of estradiol.
An androgen synthesis inhibitor is a type of drug which inhibits the enzymatic synthesis of androgens, such as testosterone and dihydrotestosterone (DHT). They include:

Estrone sulfate (E1S) is an estrogen medication and naturally occurring steroid hormone. It is used in menopausal hormone therapy among other indications. As the sodium salt, it is the major estrogen component of conjugated estrogens (Premarin) and esterified estrogens. In addition, E1S is used on its own as the piperazine salt estropipate. The compound also occurs as a major and important metabolite of estradiol and estrone. E1S is most commonly taken by mouth, but in the form of Premarin can also be taken by parenteral routes such as transdermal, vaginal, and injection.

EC508, also known as estradiol 17β-(1- -L-proline), is an estrogen which is under development by Evestra for use in menopausal hormone therapy and as a hormonal contraceptive for the prevention of pregnancy in women. It is an orally active estrogen ester – specifically, a C17β sulfonamide–proline ester of the natural and bioidentical estrogen estradiol – and acts as a prodrug of estradiol in the body. However, unlike oral estradiol and conventional oral estradiol esters such as estradiol valerate, EC508 undergoes little or no first-pass metabolism, has high oral bioavailability, and does not have disproportionate estrogenic effects in the liver. As such, it has a variety of desirable advantages over oral estradiol, similarly to parenteral estradiol, but with the convenience of oral administration. EC508 is a candidate with the potential to replace not only oral estradiol in clinical practice, but also ethinylestradiol in oral contraceptives. Evestra intends to seek Investigational New Drug status for EC508 in the second quarter of 2018.

Pregnenolone, sold under the brand name Enelone among others, is a medication and supplement as well as a naturally occurring and endogenous steroid. It is described as a neurosteroid and anti-inflammatory drug and was used in the treatment of rheumatoid arthritis and soft-tissue rheumatism in the 1950s and is no longer prescribed today, but remains available as a supplement. Pregnenolone can be taken by mouth, as a topical medication, or by injection into muscle.
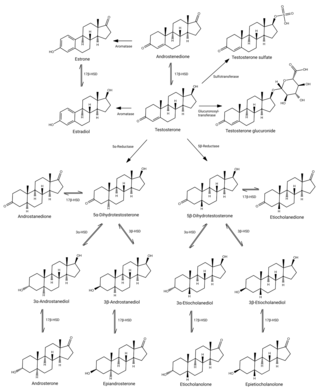
An androgen conjugate is a conjugate of an androgen, such as testosterone. They occur naturally in the body as metabolites of androgens. Androgen conjugates include sulfate esters and glucuronide conjugates and are formed by sulfotransferase and glucuronosyltransferase enzymes, respectively. In contrast to androgens, conjugates of androgens do not bind to the androgen receptor and are hormonally inactive. However, androgen conjugates can be converted back into active androgens through enzymes like steroid sulfatase.














